Translate this page into:
Bone mineral density among postmenopausal Saudi women in Riyadh city – A primary care level cross-sectional survey
Corresponding Author:
Mahmoud A Mahmoud
Department of Public Health, College of Medicine, Imam Mohammad Ibn Saud Islamic University, Riyadh City, Riyadh Province
Saudi Arabia
communitydr@hotmail.com
| How to cite this article: Mahmoud MA. Bone mineral density among postmenopausal Saudi women in Riyadh city – A primary care level cross-sectional survey. J Musculoskelet Surg Res 2019;3:350-356 |
Abstract
Objectives: This study aimed to estimate the prevalence of osteopenia and osteoporosis and to identify the associated risk factors among postmenopausal Saudi women. Methods: A community-based cross-sectional study was conducted included 501 menopausal women from 15 primary health-care centers randomly selected to be representative of various sectors of Riyadh City and three shopping malls located in Riyadh. A tested questionnaire was used to collect information about the related risk factor. Bone mineral density (BMD) of the calcaneus bone was measured using an ultrasound bone densitometer, and serum Vitamin D was measured using an autoanalyzer. BMD was classified into different categories, according to the World Health Organization classification. Descriptive statistics and logistic regression performed. Results: Mean + standard deviation age was 57.7 + 6.2 years with a range of 44–81. The prevalence of low BMD in the current study (osteopenia and osteoporosis) was 18% and 6%, respectively. The age (odds ratio [OR] = 1.07, 95% confidence interval [CI]: 1.01–1.13) and waist–hip ratio (OR = 0.94, 95% CI: 0.90–0.99) were significantly independent associated with osteopenia; also age (OR = 1.16, 95% CI: 1.09–1.24) and joint pain history (OR = 6.08, 95% CI: 2.01–18.3) were significantly independent associated with osteoporosis; and age (OR 1.08, 95% CI: 1.04–1.11) and joint pain history (1.99, 1.05–3.79) were significantly independent associated with low BMD status. Conclusions: The prevalence of low BMD among postmenopausal women was found to be lower than that reported by other studies in Saudi Arabia. Age was the crucial factor associated with Low BMD status. Further community-based studies are required to assess the community prevalence of low BMD and implement strategies to reduce the burden of its related consequences.Introduction
In the past two decades, osteoporosis has gained widespread attention as a major public health issue among policymakers and researchers.[1],[2] There is an increasing realization of the burden of diseases attributable to osteoporosis, particularly in developed countries where the aging population and increasing life expectancy have placed a greater number of people at risk of osteoporosis.[3] In 2010, low bone mineral density (BMD) was responsible for 188,000 deaths[4] Osteoporosis has been reported to cause approximately 9 million fractures annually worldwide.[5] The lifetime probability of experiencing an osteoporotic fracture in women above 50 years of age in the developed world is >40%.[6]
Although osteoporosis is asymptomatic in most cases, it can cause clinical symptoms, such as low backache, unexplained bone pain, height loss, and spinal deformities.[7]
Women in the Middle Eastern region are likely to experience a greater burden of osteoporosis.[8] An estimated 260,000 osteoporotic fractures occur annually in the Eastern Mediterranean region.[9] Despite many studies having been conducted in Saudi Arabia on the prevalence of postmenopausal osteoporosis, there is no clear picture on this issue.
The current study aimed to estimate the prevalence of, and factors associated with osteopenia and osteoporosis among postmenopausal Saudi women in Riyadh city using a community-based sampling approach and applicable community screening tool (ultrasound bone densitometer).
Materials And Methods
Study setting and participants
This community-based cross-sectional study was conducted from 2015 to 2016. The sampling technique was cluster random sample for the 15 primary health-care centers (PHCCs) and convenient sample for participants. Out of the total 116 PHCCS in the Riyadh city, 16 PHCCs were selected, four health centers from each one of the main four health sectors of the Riyadh. Only 14 were working, and two centers were under renovation, so in addition to the 14 PHCCs, the Imam University PHCC was selected to cover the northeast part of Riyadh as that area mainly occupied by the Imam University campus and served by the Imam PHCC. Participants from the three malls were 18 women had been included in PHCCs according to their residency. The final sample size was rounded off to 500 participants.[10]
Study procedures
A pretested questionnaire during a pilot study was used to collect socio-demographic details, personal habits, pertinent clinical history, obstetric history, and drug intake history. Clinical assessments were conducted in a private room to protect the privacy of the participants. Height (to the nearest 0.5 cm) was measured using a fixed stadiometer, and weight (to the nearest 500 g) was measured using a lever balance scale following the standard protocols for anthropometric measurements. Waist and Hip circumference was measured as per the World Health Organization guidelines. Blood sample for Vitamin D testing was collected from participants under strict aseptic conditions and transported under special cold boxes from the study sites to the Imam University laboratory for testing on the same day.
BMD of the left calcaneus bone was measured using an ultrasound bone densitometer (Sahara Clinical Bone Sonometer, Hologic, Bedford, MA, USA). This instrument estimates BMD from the quantitative ultrasound index and compares it to that of young, healthy, sex-matched subjects to produce a t-score. The instrument used in the current study has a predictive power of 0.72 for the area under the curve. BMD status was classified as follows: normal was a t-score of ≥−1, osteopenia was a t-score of −1 to −2.5, and osteoporosis was a t-score of <−2.5.[11]
Statistical analysis
Data entry and analysis were carried out using SPSS 17 Statistical Package for social science. A descriptive analysis was performed, and the data were described in terms of percentages or the mean and standard deviation (SD). The prevalence of osteopenia, osteoporosis status was reported along with 95% confidence intervals. The prevalence of low BMD included both osteopenia and osteoporosis, was age-standardized with reference to the national population of Saudi women, as published by the General Authority for Statistics, the Kingdom of Saudi Arabia.[12]
Chi-square tests for the categorical variables and independent samples t-tests for the continuous variables. In addition to backward stepwise logistic regression using the likelihood ratio method was performed. P < 0.05 was considered statistically significant.
Results
In the current community-based survey, a total of 501 postmenopausal Saudi women completed the interviews and laboratory testing. The mean age ± SD was 57.7 + 6.2 years.
[Table - 1] shows that the prevalence of osteopenia was 18.0% (95% confidence interval [CI]: 14.8–21.6), of osteoporosis was 6.0% (95% CI: 4.2–8.4), and of low BMD was 24.0% (95% CI: 20.4–27.9). The prevalence is progressively increased significantly with the advance of the participants' age (P < 0.001).
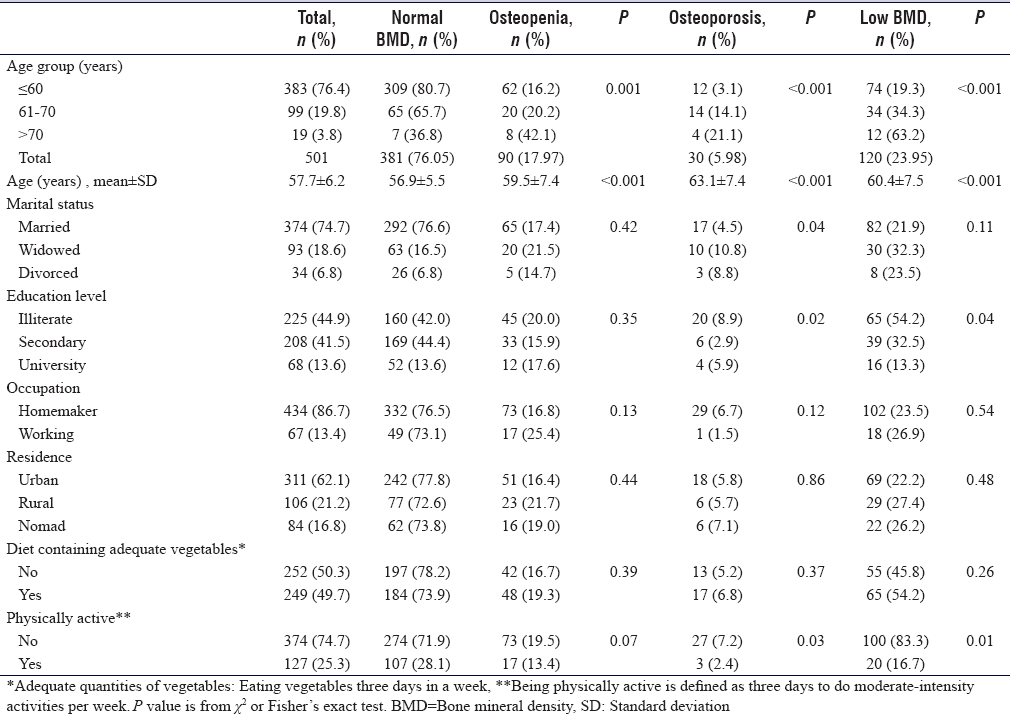
The mean age of low BMD was 60.4 ± 7.5 years significantly higher than the mean age of study participants was 57.7 + 6.2 years < 0.001.
The prevalence of low BMD is significantly higher among illiterate women compared to university graduated (54.2% vs. 44.9% P = 0.04) and the prevalence among the physically active women was significantly less in compared to the total sample (16.7% versus 25.3 P = 0.01).
Other characteristics as marital status, occupation, residence, and diet containing adequate vegetables were not associated with significant differences in the prevalence of low BMD.
[Table - 2] reveals that a history of fracture is significantly higher in participants with low BMD compared to the total participants (5.8% vs. 2.4% P = 0.005). The use of oral contraceptive pills (OCP) is significantly higher in low BMD 75.8% than total participants 67.5% (P = 0.02).
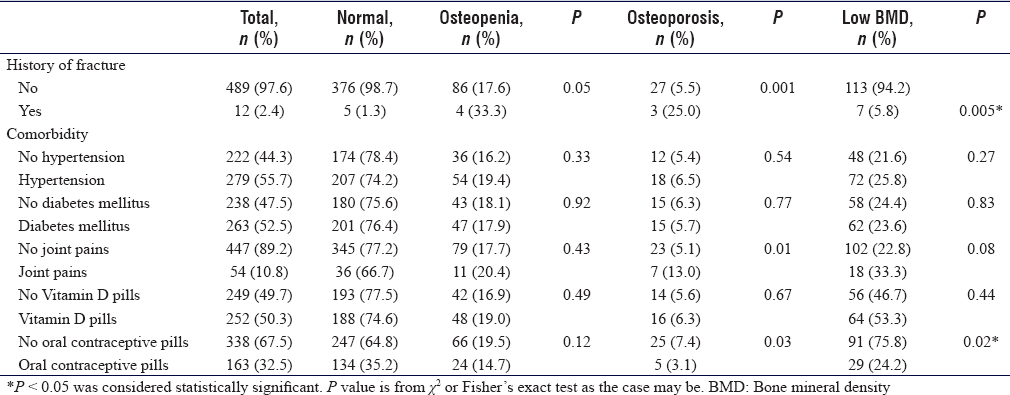
Some of the commonly reported comorbidities as hypertension (56%), diabetes (54%), thyroid disorder, and the serum Vitamin D were not associated with significant differences in the prevalence of low BMD.
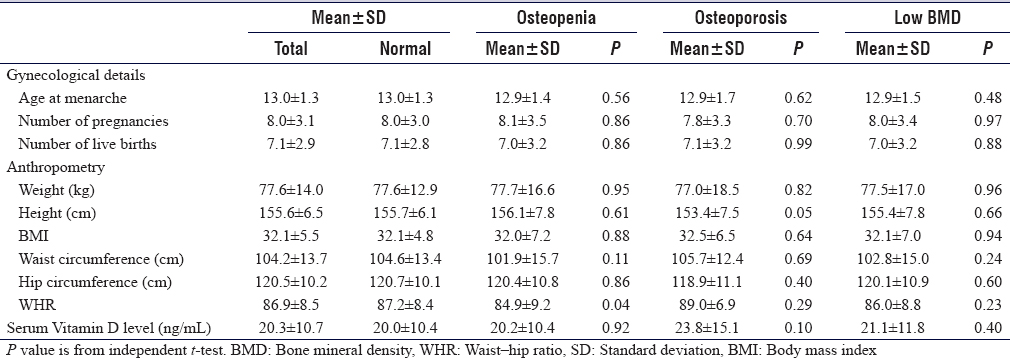
[Table - 3] shows that waist–hip ratio (WHR) was significantly associated with osteopenia, the mean ages at menarche, the mean number of pregnancies, live births, living children, and children breastfed were not statistically significant between different groups of BMDs.
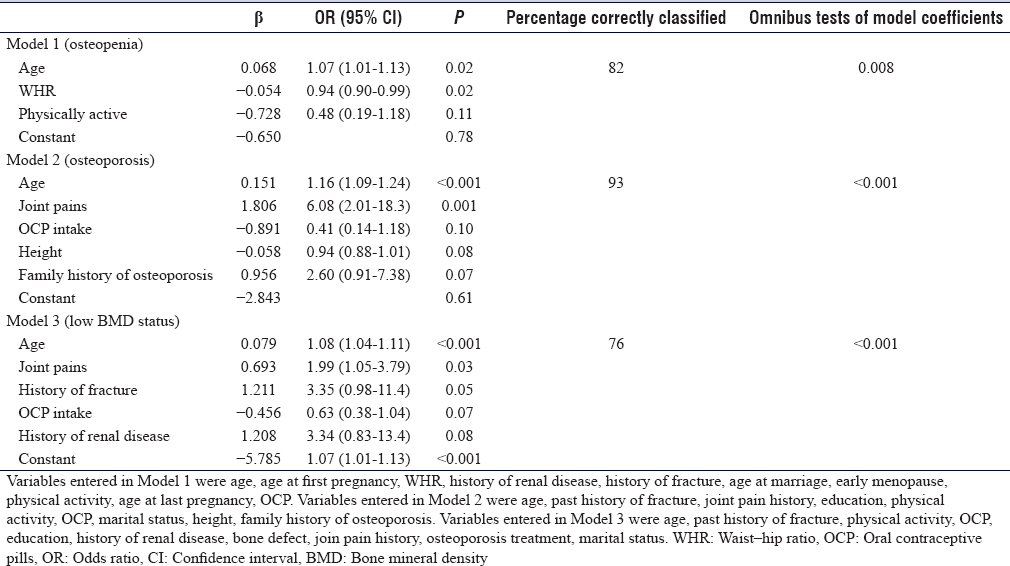
[Table - 4] shows that older age, illiteracy, physical inactivity, history of fracture, and history of renal disease were significantly associated with low BMD status in the bivariate analysis [Table - 4]. In the multiple regression analysis, the final model retained age, joint pain history, history of fracture, OCP intake, and history of renal disease. However, only age (1.08, 1.04-1.11, P < 0.001) and joint pain history (1.99, 1.05–3.79, P = 0.03) were independently associated with low BMD status.
Discussion
The prevalence of osteopenia (18%) and osteoporosis (6%) reported in the current study were lower than those reported in other studies in Saudi Arabia.
In a previous community-based cluster survey conducted in Riyadh in 2009, the prevalence of low BMD among postmenopausal women based on quantitative ultrasound (QUS) (Achilles) was 50%, which is twice as high as that reported by the current study.[13] In another study, in PHCCs of Jeddah in 2002, using dual-energy X-ray absorptiometry (DEXA) (Lunar) of the lumbar spine, the prevalence of osteopenia and osteoporosis was twice as high as that reported by the current study (50.8% and 30.4%, respectively).[14] In a study conducted among postmenopausal women recruited from shopping malls, health-care centers, and outpatient departments in 2006–2007 in the Eastern Province, the prevalence of osteopenia and osteoporosis was found to be close to that reported in the current study (30.3% and 23%, respectively), based on the QUS (Achilles) of the calcaneum.[15] In another study based on DEXA (Lunar) of the femur conducted in Jeddah during 2000–2003, the prevalence of osteopenia and osteoporosis were 57% and 7.8%, respectively, higher than the current study results.[16] Results of a study conducted among Saudi women in 2010 aged ≥50 years screened from schools, colleges, and malls in the eastern province based on the QUS (Achilles) of the calcaneum found that the prevalence of osteopenia was 31.2% and that of osteoporosis was 15.6%.[17]
Studies from other countries in the region also reported varying estimates. A Qatari study performed on 314 women aged >50 years in PHCCs in 2011–2012 reported a prevalence of 5.7% for osteopenia and 0.3% for osteoporosis based on DEXA (Lunar) of the femur.[18] A hospital-based study conducted among 292 Jordanian postmenopausal women reported a prevalence of 46.6% for osteopenia and 13% for osteoporosis based on DEXA of the femur and spine.[19]
In the USA, the prevalence of osteopenia and osteoporosis in postmenopausal white women was reported to be 54% and 30%, respectively.[20] In another American study, the prevalence of osteopenia and osteoporosis using heel sonography was found to be 34% and 3.4%, respectively.[21] In 27 European Union countries, the prevalence of osteoporosis among women aged ≥50 years ranged from 19.3%–23.4%, and the prevalence increased from 6.3% in women aged 50–54 years to 47.2% in those aged 80 years or older. These prevalence figures were based on DEXA of the hip or spine.[22]
The above findings show that there is a wide variation in the prevalence of low BMD not only between different countries but even between different parts of Saudi Arabia. Many factors influence the prevalence of low BMD. First, the age structure of the study populations is the most critical determinant of prevalence. Second, postmenopausal status is usually self-reported, and such information is not always available from other studies for comparisons; hence, the age group >50 years was used. Third, the method for measuring BMD (DEXA or ultrasound bone densitometer), studies using DEXA, and vertebral sites are likely to report higher estimates. Fourth, the sampling strategy (random, convenient, or cluster sampling) and the study setting (hospital- or community-based) greatly influence prevalence. Hospital-based studies generally produce higher estimates because of the high-risk pool of patients they select compared to the low-risk pool of participants included in a community-based study. Fifth, the variations in the distribution of risk factors, such as gynecological history, dietary differences, serum Vitamin D levels, physical activity levels, and others, also affect the prevalence of BMD.[23]
The bivariate analysis in our study showed that some factors were significantly associated with low BMD status; however, in the multivariable analysis, only age, WHR, and history of joint pains were significant. Age and history of joint pains increased the risk of having low BMD, whereas higher WHR was protective. It is a well-known fact that age is the single most important predictor of low BMD.[24] BMI or WHR has also been shown to be protective against osteoporosis in several studies.[25],[26] The presence of joint pains could be an effect of low BMD or an effect of increasing age itself, but the fact that it was retained in the stepwise model indicates that it could be independently associated with low BMD. Osteoporosis may be responsible for bone pain. Several studies in postmenopausal women conducted previously in Saudi Arabia have reported several other risk factors for osteoporosis, for example, early or late menopause, history of fractures, dietary factors, age, body weight, residence type, type 2 diabetes, physical activity, presence of comorbidities, family history, ORT, duration of lactation, and parity.[27],[28],[29],[30],[31],[32],[33],[34]
In the past few years, there has been significant interest in the role of Vitamin D in the prevention of osteoporosis and related fractures in the elderly. In the current study, serum Vitamin D levels were not associated with BMD status. There are conflicting reports of an association between serum Vitamin D and BMD. Although a few studies support the hypothesis that lower serum Vitamin D levels are associated with low BMD status,[35] many other studies do not.[29],[30],[36] The Saudi Centre for Evidence-Based Health Care does not recommend routine Vitamin D supplementation for fracture prevention in elderly patients without deficiency.[37] Similarly, a recent meta-analysis found that there was only a small benefit at the femoral neck with Vitamin D supplementation; they concluded that the widespread use of Vitamin D among adults without risk factors for osteoporosis was inappropriate as a prevention strategy based on current evidence.[38] A Cochrane review also concluded that Vitamin D supplements with or without calcium are unlikely to reduce hip fracture risks in elderly.[39] Conversely, a multinational study in 18 countries found that serum Vitamin D deficiency was prevalent between postmenopausal women with osteoporosis.[40]
Strengths and limitations
The use of ultrasound bone densitometer for the measurement of BMD in the heel in a community setting is a convenient approach, particularly for Saudi women.
The sample selected from the PHCCs was representative to Riyadh City.
The limitations in the current study included; the ultrasound bone densitometer is only a screening tool, unlike DEXA, which is the reference standard for the measurement for BMD. Therefore, the prevalence estimated in this study is expected to be lower than that reported by studies using DEXA. In addition, there is a possibility of recall bias regarding the information that was collected in the study.
Recommendations
Further community-based studies are required to assess the national-level prevalence of osteopenia and osteoporosis in the community and to implement an effective program to reduce the burden of its related consequences.
Further studies are required to identify the correction factor for different instruments so the instruments can be standardized to reflect the performance of DEXA.
Implement programs and training to increase the physicians' awareness for adopting preventive practices to screen and detect early the declining bone mass.
Conclusions
Low BMD is a pertinent health problem that affects postmenopausal women in Saudi Arabia. Effective preventive strategies are required to reduce the burden of osteoporosis and osteoporosis-related fractures. National-level prevalence studies and standardization of research methods are required to determine the burden of low BMD in Saudi Arabia accurately.
Ethics approval and consent to participate
This research has been conducted according to the ethical principles stated in the Declaration of Helsinki. Ethical clearance for this study was obtained from the Institutional Ethical Committee of Imam Mohammad Ibn Saud Islamic University (IMSIU). The results of the tests kept confidential and were communicated to the participants, and appropriate referral to specialist care was provided in cases of abnormal findings. The study was conducted with the supervision of the director of public health for the Riyadh region, local health authorities and Imam Medical center administrators.
Financial support and sponsorship
This study was supported by IMSIU, Riyadh, Saudi Arabia.
Conflicts of interest
There are no conflicts of interest.
Author's contributions
The study was conceived and implemented by MAM. The manuscript was written and finalized by MAM. The author has critically reviewed and approved the final draft and is responsible for the content and similarity index of the manuscript.
| 1. | International Osteoporosis Foundation. Annual Report, 2016. International Osteoporosis Foundation; 2016. Available from: https://www.iofbonehealth.org/about-us/annual-report. [Last accessed on 2019 Jan 01]. [Google Scholar] |
| 2. | World Health Organization. The World Health Report 2004: Changing History. Geneva: World Health Organization; 2004. [Google Scholar] |
| 3. | Reginster JY, Burlet N. Osteoporosis: A still increasing prevalence. Bone 2006;38:S4-9. [Google Scholar] |
| 4. | Sànchez-Riera L, Carnahan E, Vos T, Veerman L, Norman R, Lim SS, et al. The global burden attributable to low bone mineral density. Ann Rheum Dis 2014;73:1635-45. [Google Scholar] |
| 5. | Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726-33. [Google Scholar] |
| 6. | Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002;359:1929-36. [Google Scholar] |
| 7. | Kanis JA. WHO Technical Report. United Kingdom: University of Sheffield; 2007. [Google Scholar] |
| 8. | Baddoura R, Hoteit M, El-Hajj Fuleihan G. Osteoporotic fractures, DXA, and fracture risk assessment: Meeting future challenges in the Eastern Mediterranean region. J Clin Densitom 2011;14:384-94. [Google Scholar] |
| 9. | World Health Organization. WHO Scientific Group on the Assessment of Osteoporosis at Primary Health Care Level. Geneva: World Health Organization; 2007. Available from: http://www.who.int/chp/topics/Osteoporosis.pdf. [Last accessed on 2019 Feb 20]. [Google Scholar] |
| 10. | El-Desouki MI. Osteoporosis in postmenopausal Saudi women using dual x-ray bone densitometry. Saudi Med J 2003;24:953-6. [Google Scholar] |
| 11. | Krieg MA, Cornuz J, Ruffieux C, Van Melle G, Büche D, Dambacher MA, et al. Prediction of hip fracture risk by quantitative ultrasound in more than 7000 Swiss women and gt; or=70 years of age: Comparison of three technologically different bone ultrasound devices in the SEMOF study. J Bone Miner Res 2006;21:1457-63. [Google Scholar] |
| 12. | General Authority for Statistics, Kingdom of Saudi Arabia Population estimates; 2015. Available from: https://www.stats.gov.sa/en/43. [Last accessed on 2019 May 18]. [Google Scholar] |
| 13. | AlQuaiz AM, Kazi A, Tayel S, Shaikh SA, Al-Sharif A, Othman S, et al. Prevalence and factors associated with low bone mineral density in Saudi women: A community based survey. BMC Musculoskelet Disord 2014;15:5. [Google Scholar] |
| 14. | Al-Raddadi R. The Prevalence of Osteoporosis and Its Associated risk Factors Among Saudis 30 years of Age and above in Jeddah City, Saudi Arabia (Thesis); 2010. [Google Scholar] |
| 15. | Al-Habdan IM, Sadat-Ali M, Al-Muhanna FA, Al-Elq AH, Al-Mulhim AA. Bone mass measurement using quantitative ultrasound in healthy Saudi women. A cross-sectional screening. Saudi Med J 2009;30:1426-31. [Google Scholar] |
| 16. | Ardawi MS, Maimany AA, Bahksh TM, Nasrat HA, Milaat WA, Al-Raddadi RM. Bone mineral density of the spine and femur in healthy Saudis. Osteoporos Int 2005;16:43-55. [Google Scholar] |
| 17. | Sadat-Ali M, Al-Elq A, Al-Habdan I, Al-Mohanna FA, Al-Mulhim AA. Quantitative ultrasound (QUS) of the OS calcis in Saudi women: Defining Saudi reference value for the diagnosis of low bone mass. Arch Osteoporos 2010;5:139-44. [Google Scholar] |
| 18. | Gerber LM, Bener A, Al-Ali HM, Hammoudeh M, Liu LQ, Verjee M. Bone mineral density in midlife women: The study of women's health in Qatar. Climacteric 2015;18:316-22. [Google Scholar] |
| 19. | El-Heis MA, Al-Kamil EA, Kheirallah KA, Al-Shatnawi TN, Gharaibia M, Al-Mnayyis A. Factors associated with osteoporosis among a sample of Jordanian women referred for investigation for osteoporosis. East Mediterr Health J 2013;19:459-64. [Google Scholar] |
| 20. | Kanis JA, Johnell O, Oden A, Jonsson B, De Laet C, Dawson A. Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone 2000;27:585-90. [Google Scholar] |
| 21. | Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: Results from the national osteoporosis risk assessment. JAMA 2001;286:2815-22. [Google Scholar] |
| 22. | Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 2013;8:136. [Google Scholar] |
| 23. | Greer W, Ahmed M, Rifai A, Sandridge AL. Exploring the extent of postmenopausal osteoporosis among Saudi Arabian women using dynamic simulation. J Clin Densitom 2008;11:543-54. [Google Scholar] |
| 24. | Siris ES, Brenneman SK, Barrett-Connor E, Miller PD, Sajjan S, Berger ML, et al. The effect of age and bone mineral density on the absolute, excess, and relative risk of fracture in postmenopausal women aged 50-99: Results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int 2006;17:565-74. [Google Scholar] |
| 25. | Morin S, Tsang JF, Leslie WD. Weight and body mass index predict bone mineral density and fractures in women aged 40 to 59 years. Osteoporos Int 2009;20:363-70. [Google Scholar] |
| 26. | Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 2007;92:1640-6. [Google Scholar] |
| 27. | World Health Organization. Noncommunicable Diseases and Mental Health Cluster. WHO STEPS surveillance manual: The WHO STEPwise approach to chronic disease risk factor surveillance / Noncommunicable Diseases and Mental Health, World Health Organization. World Health Organization. 2005. Available from:https://apps.who.int/iris/handle/10665/43376 [Last accessed on 2019 Oct 01]. [Google Scholar] |
| 28. | Addar M, El Desouki M, Babay Z. Correlates of age at menopause and osteoporosis in Saudi women. Clin Exp Obstet Gynecol 2005;32:135-7. [Google Scholar] |
| 29. | Alsaif MA, Khan LK, Alhamdan AA, Alorf SM, Al-Othman AM, Makki RJ. Dietary factors contributing to osteoporosis among post menopausal Saudi women. J Appl Sci 2007;7:2776-81. [Google Scholar] |
| 30. | Alissa EM, Alnahdi WA, Alama N, Ferns GA. Relationship between nutritional profile, measures of adiposity, and bone mineral density in postmenopausal Saudi women. J Am Coll Nutr 2014;33:206-14. [Google Scholar] |
| 31. | Al-Maatouq MA, El-Desouki MI, Othman SA, Mattar EH, Babay ZA, Addar M. Prevalence of osteoporosis among postmenopausal females with diabetes mellitus. Saudi Med J 2004;25:1423-7. [Google Scholar] |
| 32. | Aboel-Fetoh NM, Abukanna A, Hamad A, Ibn Idris HO, Adlan N, Olama SM. Risk factors for osteoporosis in women forty years and above in Arar City, Kingdom of Saudi Arabia (KSA). Med Sci Clin Res 2015;3. DOI: http://dx.doi.org/10.18535/jmscr/v3i9.25. Available from: http://jmscr.igmpublication.org/home/index.php/archive/237-risk-factors-for-osteoporosis-in-women -forty-years-and-above-in-arar-city-kingdom-of-saudi-arabia-ksa #1-abstract. [Last accessed on 2019 May 16]. [Google Scholar] |
| 33. | Al-Raddadi RM, Al Kadi HA, Hakim FF, Ardawi MS. The effect of parity and lactation on bone mineral density among Saudi women, Jeddah. Montreal, Canada: 3RD WORLD CONGRESS, on Controversies, Debates & Consensus, in Bone, Muscle & Joint Diseases; 2015. Available from: https://www.researchgate.net/publication/275831600. [Last accessed on 2019 Sep 30]. [Google Scholar] |
| 34. | Sadat-Ali M, Al-Habdan I, Al-Mulhim AA, El-Hassan AY. Effect of parity on bone mineral density among postmenopausal Saudi Arabian women. Saudi Med J 2005;26:1588-90. [Google Scholar] |
| 35. | Sadat-Ali M, Al Elq AH, Al-Turki HA, Al-Mulhim FA, Al-Ali AK. Influence of vitamin D levels on bone mineral density and osteoporosis. Ann Saudi Med 2011;31:602-8. [Google Scholar] |
| 36. | Alkhenizan A, Mahmoud A, Hussain A, Gabr A, Alsoghayer S, Eldali A. The relationship between 25 (OH) D levels (Vitamin D) and bone mineral density (BMD) in a Saudi population in a community-based setting. PLoS One 2017;12:e0169122. [Google Scholar] |
| 37. | The Saudi Centre for Evidence Based Health Care. Clinical practice guideline on the role of vitamin D, calcium and exercise in fracture prevention in elderly. Ministry of Health, Riyadh. 2014. Available from: https://www.researchgate.net/publication/288493490_Clinical_Practice_Guideline_on_the_Role_of_Vitamin_D_Calcium_and_Exercise_in_Fracture_Prevention_in_Elderly [Last accessed on 2019 May 16]. [Google Scholar] |
| 38. | Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: A systematic review and meta-analysis. Lancet 2014;383:146-55. [Google Scholar] |
| 39. | Avenell A, Gillespie WJ, Gillespie LD, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst Rev 2009;(2):CD000227. [Google Scholar] |
| 40. | Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, et al. The prevalence of Vitamin D inadequacy amongst women with osteoporosis: An international epidemiological investigation. J Intern Med 2006;260:245-54. [Google Scholar] |
Fulltext Views
3,091
PDF downloads
1,257





