Translate this page into:
Giant cell tumor in the proximal phalanges of the hand: A report of two cases treated with a nonbiological construct
2 College of Medicine, Alfaisal University, Riyadh, Saudi Arabia
Corresponding Author:
Rajeev Pant
MBC 77, P.O. Box: 3354, Riyadh 11211
Saudi Arabia
raj.pant@gmail.com
| How to cite this article: Altayeb MA, Shaheen MF, Abduljawad SM, Pant R. Giant cell tumor in the proximal phalanges of the hand: A report of two cases treated with a nonbiological construct. J Musculoskelet Surg Res 2021;5:62-66 |
Abstract
Giant cell tumor of bone (GCT-B) accounts for approximately 5% of all primary bone tumors and 20% of all benign bone tumors. Its occurrence in the hand is rare, accounting for <2% of cases. In the Rizzoli study covering the 50 years between 1947 and 1997, of the 900 patients with GCT-B, only 8 (0.9%) had GCT in the small bones of the hand and no phalangeal lesions were observed. A five-decade review of GCT of bone from the Mayo Clinic came up with just five cases of GCT in the phalanges (and 13 overall in the small bones of the hand). Our experience at the King Faisal Specialist Hospital covered the period between 1985 and 2020. During these 35 years, we saw 350 patients with GCT-B, of whom only 2 (<1%) patients presented with phalangeal lesions. Both patients were treated by excision of the involved phalanx and nonbiological reconstruction using a Kirschner wire and cement spacer. A literature review and management options for this rare presentation site in GCT-B are discussed.
Introduction
Giant cell tumors of bone (GCT-B) are benign neoplastic bone lesions with aggressive behavior, accounting for 5% of all primary bone tumors and 20% of benign bone tumors.[1],[2] The lesion is classically juxta-articular at the end of long bones in the age group of 20–40 years with the mean age of 32 years. On the hand, it tends to present earlier with a mean age at presentation of 22 years (that is less than the average occurrence age of conventional GCT).[3],[4] The most common location for GCTs is the distal femur and the proximal tibia, which together constitute 55% of cases. Other locations include the distal radius (10%–12%), sacrum (4%–9%), proximal humerus (4%–8%), proximal femur (4%), and less frequently, the vertebral bodies (2.5%).[5] However, its occurrence in the bones of the hands is an infrequent entity, accounting for only 2% of all GCTs,[5],[6] and it is even rarer in the phalanges of the hands. Biscaglia reported a 0.9% occurrence in the hands in their review of 900 cases.[7] Averill reported an incidence of 2% GCT in the hands out of 1228 cases.[4] In a review by Patel et al. of 2400 cases, fifty cases (2%) were in the phalanges of the hand.[8] Athanasian, in his data over 50 years from the Mayo Clinic, had just five patients with GCT in the phalanges.[9] From a collection of series, in Turcott's report, of 1299, only 11 (0.84%) cases occurred in the phalanges.[5]
We report on two patients with GCT-B of the phalanges. The first case presented with GCT-B in the proximal phalanx of the thumb and the second case with the tumor in the proximal phalanx of the index finger. In both patients, the lesions were radiologically Campanacci III. They were treated by total resection of the involved phalanx and nonbiological reconstruction using a Kirschner (K) wire and cement spacer. Both patients remained disease-free at 9 and 7 years, respectively, post index procedure. A literature review and management options for this rare presentation site in GCT-B are discussed.
Case Reports
Case 1
A 46-year-old female presented in February 2011, with pain and swelling in the right thumb. She had a significant local soft-tissue swelling. She stated that her symptoms started 2 months before her first presentation to a local hospital. She had an outside magnetic resonance image (MRI) done in January 2011 before referral to us. Her radiographs showed an expansile, lytic, destructive lesion of the proximal phalanx of the right thumb. The proximal 2/3rd of the phalanx was completely destroyed with just a small mottled distal remnant [Figure - 1]a and [Figure - 1]b. MRI studies, in addition to corroborating the plain film findings, also revealed the encasement of the flexor pollicis longus tendon [Figure - 2]a, [Figure - 2]b, [Figure - 2]c, [Figure - 2]d. There were no fluid levels to suggest an aneurysmal bone cyst (ABC), and the solitary lesion with no corroborative changes in the bone profile plus normal parathyroid hormone (PTH) levels ruled out the possibility of this being a Brown's tumor. The clinical/radiological picture was consistent with a diagnosis of GCT-B. Routine laboratory tests were normal, and her chest computed tomography (CT) scan was negative for any suspicious lesions. The bone scan was deferred, pending a biopsy as bone metastasis from a benign GCT would not be expected. A core biopsy was consistent with this being a benign GCT-B. She underwent formal surgical intervention in March, which involved a thorough curettage and excision of the remnant phalanx. No adjuvant could be used as the tumor had completely destroyed the bone and extended into the soft tissues. The long extensor/flexor tendons were both preserved, and besides the meticulous curettage, we had to depend on copious irrigation of the wound with saline and hydrogen peroxide. The ensuing defect was reconstructed using a K-wire plus cement construct to maintain length, achieving double fusion at the interphalangeal (IP) joint and metacarpophalangeal (MP) joint.
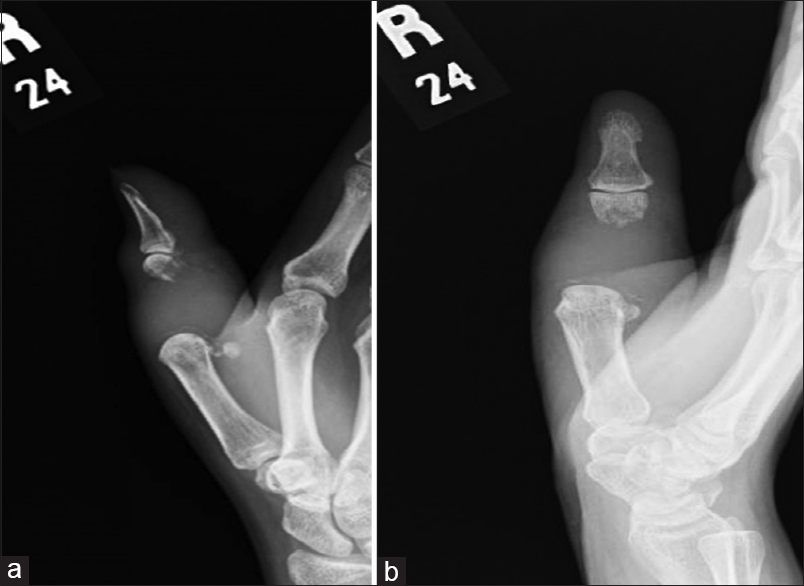 |
| Figure 1: (a and b) Right thumb, anterior-posterior, and lateral X-ray views. There is a subarticular expansile destructive lytic lesion involving the proximal first phalanx with evidence of soft-tissue involvement |
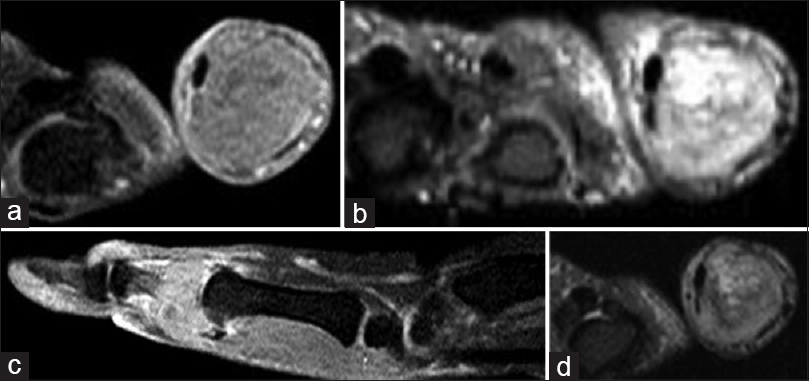 |
| Figure 2: (a-d) Magnetic resonance image of the right thumb. There is an expansile bony lesion involving the proximal phalanx. It demonstrates intermediate to high-signal intensity on T2-weighted images and inhomogeneous moderate enhancement in postcontrast images. The lesion shows encasement of the flexor pollicis longus tendon |
The final histopathology report was consistent with a GCT-B. She was followed up and imaged postoperatively as per our tumor protocol at 3-month intervals for the first 2 years, at 4-monthly intervals in year 3, at 6 monthly intervals in years 4 and 5, and annually after that. Her last follow-up was in May 2019. She had no local recurrence (LR), remained disease-free, and the nonbiological construct remained intact. Due to the COVID-19 situation, she was contacted through the virtual clinic in May 2020 when she reported no changes to her situation either with regard to the appearance of any new swellings or alteration in the functional ability of her hand. Although she gave consent for her case to be published, she was reluctant about her clinical photographs to be presented.
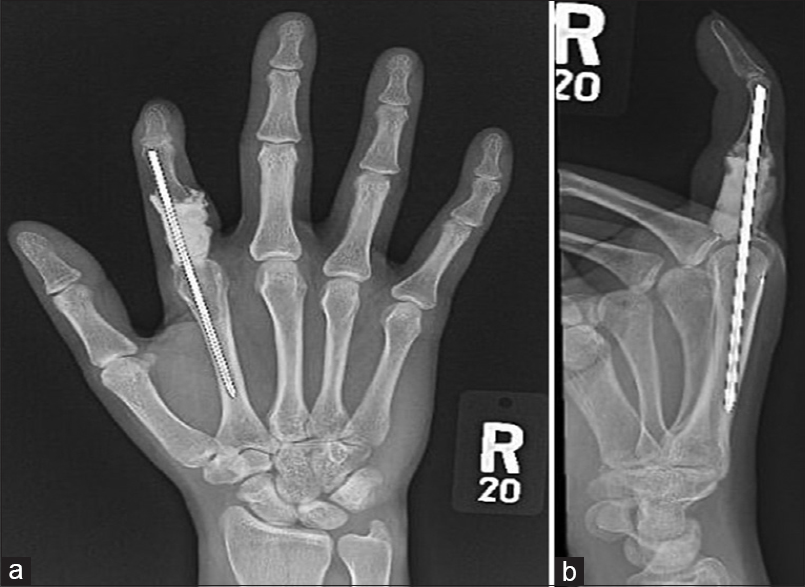
The patient inevitably has no flexion at the thumb interphalangeal joint but remains functional with a musculoskeletal tumor society (MSTS) score of 88.5 and a shortened disabilities of the arm, shoulder, and hand (QuickDASH) score of 15.9% [Figure - 3]a and [Figure - 3]b.
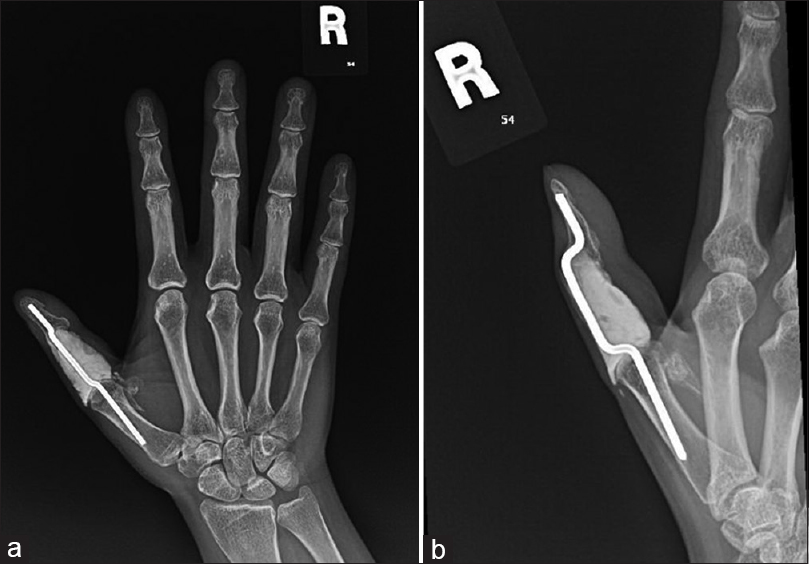 |
| Figure 3: (a and b) X-ray of the right hand during the last follow-up. No hardware-related complication, no radiographic evidence of local tumor recurrence |
Case 2
A 23-year-old female patient presented to our clinic in November 2013. Four months earlier, she had noticed a progressive swelling of her right index finger associated with pain. On examination, there was marked swelling and associated tenderness of the proximal phalanx of the right index finger, limiting her range of motion (ROM) at the contiguous proximal interphalangeal (PIP) and MP joints. The skin was intact. Her radiographs showed an expansile lytic bone lesion destroying the proximal 3/4th of the affected phalanx with an obvious extension of the tumor into the soft tissues. Based on the clinical presentation and radiological appearance, the differential diagnosis was between an ABC, Brown's tumor, and a GCT-B, Campanacci III [Figure - 4]a and [Figure - 4]b. The absence of fluid levels on the MRI made a diagnosis of ABC unlikely. A normal bone profile and no elevation of PTH levels ruled out a Brown's tumor. The working diagnosis then was of a GCT-B. Chest imaging was negative for metastases, and as with the previous case, on a working diagnosis of GCT-B, we did not feel a bone scan was justified. That same month, she underwent an open biopsy and frozen section confirming a diagnosis of GCT-B, and we proceeded to a radical phalangectomy and meticulous curettage under the same anesthetic. Once again, tumor extension into the soft tissues precluded the use of any adjuvant modality, with one having to depend solely on the thoroughness of the curettage and irrigation of the wound with hydrogen peroxide. The reconstruction technique was the same as in the previous case using a cement spacer and K-wire for double fusion.
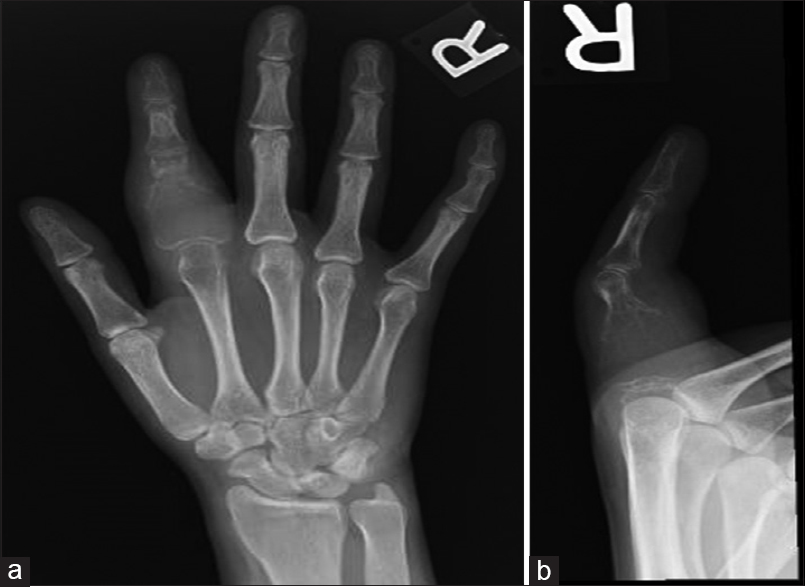 |
| Figure 4: (a and b) Anterior-posterior and lateral view of the right hand index finger. There is an expansile lytic lesion at the proximal phalanx with a thin cortical outline and soft-tissue swelling |
Ten months postoperatively, in September 2014, she presented with a soft-tissue recurrence [Figure - 5]a and [Figure - 5]b. This was dealt with by an exchange of the cement and K-wire combined with excision of the LR. She has remained disease-free locally thereafter, and her chest imaging has remained clear. The postoperative follow-up protocol was identical to the previous case but with the 3-monthly reviews being recommenced from the date of her last LR [Figure - 6]a and [Figure - 6]b. Due to COVID-19, the patient was contacted through the virtual clinic in April 2020 when she had no new issues or complaints.
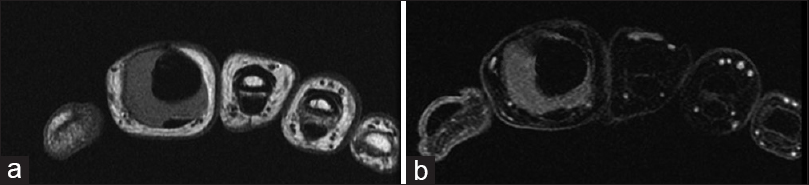 |
| Figure 5: (a and b) Magnetic resonance image of the right index finger showing evidence of local tumor recurrence surrounding the nonbiological construct |
 |
| Figure 6: (a and b) X-ray of the right index finger during the last follow-up. No hardware-related complication and no radiological evidence of tumor recurrence |
The patient obviously has no flexion at the index finger MP/PIP joints but retains pulp-to-pulp between thumb and the other digits, and pulp-to-side pinch ability with the thumb and index finger with an MSTS functional score of 82.8 and QuickDASH score of 29.5% [[Figure - 7]a, [Figure - 7]b, [Figure - 7]c.
 |
| Figure 7: (a-c) Clinical photographs showing good occupational performance |
Discussion
Phalangeal GCT-Bs are aggressive lesions. Both of our cases were Campanacci III on presentation. This appears to be consistent with the described behavior of GCT-B at this location being more destructive than at other locations. Lesions at this site rarely present as Campanacci I.[8],[9] Ropares, in his review of 214 cases, found three cases of GCT-B of the phalanx, all of whom presented as stage III.[10] They usually present with swelling, pain, and limited joint motion. The digital location results in the pathology being noticed earlier, with an average duration of symptoms of about 3.6 months.[4],[5],[10] Our patients noticed a progressive swelling at 2 and 4 months, respectively, before seeking medical attention.
There is no agreement about the management of GCT in the phalanges. There are multiple methods of management of GCT in general, these include but are not limited to curettage and bone grafting (with the use of adjuvants), marginal excision (phalangectomy), or wide excision (amputation). Curettage alone has a high recurrence rate (up to 90%), and most recurrences end up requiring an amputation.[4] The second case in our report had a recurrence 10 months after the first procedure, which we managed with a revision wide excision.
A thorough curettage supplemented with the use of a dental burr plus the use of augmentation agents is believed to decrease the risk of LR when dealing with GCT-B. To minimize the risk of LR, this curettage is then further augmented by the use of adjuvant agents, which may be chemical (phenol and alcohol), thermal (argon beam), or cryotherapy (liquid nitrogen). Hydrogen peroxide wash too has been mentioned as being of potential value in minimizing LR risk. However, these agents are effective only if the tumor per se has first been adequately removed/resected.[11],[12] In both of our cases, we resected the involved phalanx, but the surrounding soft-tissue extension precluded the use of any adjuvant therapy. A meticulous bone and soft-tissue resection and thorough curettage (plus hydrogen peroxide wash) were the only procedural options open to us in reducing the LR rate. Phalangeal lesions inevitably jeopardize the contiguous joints. When both the proximal and distal joints of the phalanx are involved, a double arthrodesis is inevitably the only option. No matter what form of reconstruction is used, some form of fixation (wires or screws) would be mandatory to hold the construct in place. Theoretically, this would involve the risk of tumor cell seeding with the use of the implant placement in the proximal and distal bones, but implant placement is unavoidable for the above-mentioned reasons. In any event, this risk would not be greater than the risk of LR, which is why a thorough tumor clearance has to be of the utmost priority.
The usage of bone cement in GCT has been well described, its advantage, including the theory of the potential for obtaining “peripheral kill” due to its exothermic reaction when it sets. Another advantage is in providing a “visual contrast” radiologically to detect any early recurrence. We intentionally chose to use bone cement as a component of our nonbiological construct for both the said reasons as well as for providing immediate stability. Fusion using bone graft (autograft or allograft) has been described in phalangeal GCT-B with comparable functional outcomes.[13],[14],[15] When using bone grafting, it is necessary to wait for healing and having the risks of union complications. This would not give the immediate stability provided by bone cement, and if bone graft resolved, this would be thought radiologically as early recurrence.
In similar lesions, where both MP and PIP joints are involved, Beltrami described a new option with a three-dimensional (3D) printing technique and had an excellent result. Titanium alloy designed implant was printed based on a CT scan of the contralateral proximal phalanx. This, Beltrami's option, needs the facilities of 3D printing, which may not be readily available at most centers. In his report, he used denosumab as neoadjuvant therapy.[16] The use of denosumab in GCT is beyond the scope of our report. In general, using denosumab is acceptable in advanced GCT of bone with the intent to convert joint resection surgery to joint sparing surgery.[17]
The functional and cosmetic appearance both need to be taken into consideration when undertaking a double fusion after resection of a phalanx. From a functional perspective, a double fusion with the MP joint fused at 20° and the PIP joint (PIPJ) at 40° would allow pulp-to-palm apposition when making a fist, but it would be a fixed and ungainly deformity at all other times. Cosmetically, it would be unacceptable though functionally definitively better. In our case, with only one digit involved, we felt that there would be no significant compromise of functional ability by “fusing” in extension while maintaining cosmesis. Our scoring in terms of acceptability is a testament to the same.
When only one joint, usually the MP joint, of the proximal phalanx requires resection, other reconstruction options have been described to fend off joint fusion. Ansari et al. described using a silicon arthroplasty implant with fibular autografting after resection of the proximal part of the proximal phalanx because of GCT in the index finger. A good ROM was achieved and no LR after 18 months of follow-up.[18] Spiro et al. used osteochondral autograft from the lateral femoral condyle to reconstruct the MP joint in the proximal phalanx of the index finger after GCT excision from the base of the phalanx with a stable, satisfactory ROM and no signs of recurrence in 2-year follow-up.[19] An osteochondral phalangeal allograft would function effectively as a replacement for the excised phalanx after ligamentous reattachment. This option was not open to us as osteochondral allografts are not available in our bone bank.
The use of vascularized metatarsophalangeal joint transfer to the MP joint after resection the base of index phalanx due to GCT of bone was described by Kanaya.[20] Smith and Millender described another technique of toe transfer. They resected the middle phalanx of the little finger after recurrent GCT, and the proximal phalanx from the little toe was transferred in its place, providing a good ROM at the PIPJ and no LR after 1 year.[21]
Conclusion
Phalangeal GCT of bone is challenging. There are no best treatment options, and the aim is to save the digit and function besides lowering the risk of recurrence. We have achieved good control even in the incidence of recurrences with precise surgical excision and using nonbiological constructs.
We describe a K-wire plus intercalary cement nonbiological construct for managing such pathology. We believe that this is an option when amputation is not ideal (as with the involvement of the thumb) or when the patient is not accepting ablative surgery (as in our second case). In these circumstances, an arthrodesis would need to be considered either with bone graft (autograft + allograft) or using a nonbiological construct as described by this report.
The average construct survival in our two cases was 8 years and counting. The MSTS functional scoring was 88.5 and 82.8, and the QuickDASH score was 15.9% and 29.5%, respectively.
Ethical consideration
The approval for publication was obtained from the institutional office of research on January 2020, reference number: RAC # 2200001.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal the identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
There are no conflicts of interest.
Authors' contribution
RP, MFS, and MAA conceived and designed the study; RP, MAA, and SMA conducted the literature search; RP and MAA have contributed with manuscript preparation, editing, and writing the initial and final draft. All authors have critically reviewed and approved the final draft and are responsible for the manuscript's content and similarity index.
| 1. | Thomas DM, Keith MS. Giant cell tumour of bone. Curr Opin Oncol 2009;21:338-44. [Google Scholar] |
| 2. | Werner M. Giant cell tumour of bone: Morphological, biological and histogenetical aspects. Int Orthop 2006;30:484-9. [Google Scholar] |
| 3. | Yin Y, Gilula LA, Kyriakos M, Manske P. Giant-cell tumor of the distal phalanx of the hand in a child. Clin Orthop Relat Res 1995:200-7. [Google Scholar] |
| 4. | Averill RM, Smith RJ, Campbell CJ. Giant-cell tumors of the bones of the hand. J Hand Surg Am 1980;5:39-50. [Google Scholar] |
| 5. | Turcotte RE. Giant cell tumor of bone. Orthop Clin North Am 2006;37:35-51. [Google Scholar] |
| 6. | Unni KK. Dahlin's Bone Tumours: General Aspects and Data on 11087 Cases. 5th ed. Philadelphia: Lippincott-Raven; 1996. p. 263-83. [Google Scholar] |
| 7. | Biscaglia R, Bacchini P, Bertoni F. Giant cell tumor of the bones of the hand and foot. Cancer 2000;88:2022-32. [Google Scholar] |
| 8. | Patel MR, Desai SS, Gordon SL, Nimberg GA, Sclafani SJ, Vigorita VJ, et al. Management of skeletal giant cell tumors of the phalanges of the hand. J Hand Surg Am 1987;12:70-7. [Google Scholar] |
| 9. | Athanasian EA, Wold LE, Amadio PC. Giant cell tumors of the bones of the hand. J Hand Surg Am 1997;22:91-8. [Google Scholar] |
| 10. | Ropars M, Kaila R, Cannon SR, Briggs TW. Primary giant cell tumours of the digital bones of the hand. J Hand Surg Eur Vol 2007;32:160-4. [Google Scholar] |
| 11. | Algawahmed H, Turcotte R, Farrokhyar F, Ghert M. High-speed burring with and without the use of surgical adjuvants in the intralesional management of giant cell tumor of bone: A systematic review and meta-analysis. Sarcoma 2010;2010:1-5. [Google Scholar] |
| 12. | Blackley HR, Wunder JS, Davis AM, White LM, Kandel R, Bell RS. Treatment of giant-cell tumors of long bones with curettage and bone-grafting. J Bone Joint Surg Am 1999;81:811-20. [Google Scholar] |
| 13. | Reichert P, Kowalski P, Gosk J. The giant cell tumour of the proximal phalanx of the thumb treated by a 2-stage operation. Acta Orthop Traumatol Turc 2017;51:425-8. [Google Scholar] |
| 14. | Saikia KC, Bhuyan SK, Goswami S, Bora A. Rare site giant cell tumors: Report of two cases on phalanges of the finger and review of literature. J Orthop Traumatol 2009;10:193-7. [Google Scholar] |
| 15. | Agrawal A. Recurrent giant cell tumor of the phalanx: A rare occurrence. J Ortho Traumatol Rehabil 2017;9:59-61. [Google Scholar] |
| 16. | Beltrami G. Custom 3D-printed finger proximal phalanx as salvage of limb function after aggressive recurrence of giant cell tumour. BMJ ase Rep 2018;2018:1-4. [Google Scholar] |
| 17. | van der Heijden L, Dijkstra PDS, Blay JY, Gelderblom H. Giant cell tumour of bone in the denosumab era. Eur J Cancer 2017;77:75-83. [Google Scholar] |
| 18. | Ansari MT, Kotwal PP, Rao S. Reconstruction with fibular autograft and silicone implant arthroplasty after resection of giant-cell tumour of the proximal phalanx: A case report with 18-month follow-up. Musculoskelet Surg 2014;98:153-7. [Google Scholar] |
| 19. | Spiro AS, Pogoda P, Amling M, Meenen NM, Zustin J, Rueger JM, et al. Giant cell tumour of bone: Reconstruction of the index metacarpophalangeal joint with an osteochondral graft from the lateral femoral condyle. J Plast Reconstr Aesthet Surg 2013;66:729-32. [Google Scholar] |
| 20. | Kanaya K, Wada T, Kitajima K, Yamashita T. Vascularized metatarsophalangeal joint transfer for giant cell tumor of the proximal phalanx of the hand. Plast Reconstr Surg 2008;121:354-5. [Google Scholar] |
| 21. | Smith JA, Millender LH. Treatment of recurrent giant-cell tumor of the digit by phalangeal excision and toe phalanx transplant: A case report. J Hand Surg Am 1979;4:164-7. [Google Scholar] |
Fulltext Views
4,491
PDF downloads
1,706





