Translate this page into:
Ultrasound to guide nerve transfer healing and muscle re-innervation
*Corresponding author: J. Terrence Jose Jerome, Department of Orthopaedics, Hand and Reconstructive Microsurgery, Olympia Hospital and Research Centre, Trichy, Tamil Nadu, India. terrencejose@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jerome JTJ. Ultrasound to guide nerve transfer healing and muscle re-innervation. J Musculoskelet Surg Res. 2024;8:426-9. doi: 10.25259/JMSR_263_2024
Abstract
Nerve transfer and muscle re-innervation are critical components for restoring function and managing brachial plexus injuries. This technical note highlights the role of ultrasound in assessing the success of a nerve transfer after the surgical procedure. By visualizing the nerve transfer site and evaluating muscle response to stimulation, ultrasound can help confirm that the transferred nerve is healing and re-innervating the target muscle. This report highlights the effectiveness of ultrasound in the early diagnosis and monitoring of nerve re-innervation and muscle recovery.
Keywords
Biceps contraction
Distal nerve transfer
Healing
Muscle re-innervation
Ultrasound
INTRODUCTION
There are many ways to assess muscle re-innervation following brachial plexus nerve transfers, which include electromyography (EMG), nerve conduction studies (NCS), and clinical evaluation using the Medical Research Council (MRC) grading.[1-5] With EMG, the re-innervating muscle exhibits motor unit potentials (MUPs) with longer duration and larger amplitude, often accompanied by increased polyphasia. The percentage of polyphasic MUPs serves as an indicator of the degree of ongoing spontaneous re-innervation. Over time, re-innervated MUPs gradually lose their polyphasic morphology while retaining their larger amplitude and, to a lesser extent, their longer duration. The chronic morphology of MUPs indicates successful muscle reinnervation.[1,3,5] In the NCS, the re-innervating muscles have an increase in compound motor action potential. It is challenging to compare these findings with clinical recovery, and it depends on the technical expertise involved.
Clinical examination findings of re-innervation of the muscle will take several weeks to several months. The nerve regeneration occurs at 1 mm/day, and it takes a minimum of 3–6 months to appreciate elbow flexion.[6] Re-innervation of the biceps becomes efficient and functional when a sufficient number of axons reach their target, along with appropriate motor afferent re-innervation, ensuring adequate muscle strength and elbow flexion. Furthermore, muscle tenderness is an indicator of muscle re-innervation, which can be appreciated by pressing the biceps muscle. This deep palpation tenderness is a good prognostic sign predicting expectant muscle contractions. It takes approximately 3 months to have biceps tenderness, 4 months to notice visible contraction, and 12 months to perceive functional movement.
Assessing nerve pathology can be effectively achieved using ultrasound. In a transverse section, a normal nerve displays small hypoechoic areas separated by hyperechoic septa, creating a “honeycomb-like” appearance.[2] These hypoechoic areas represent nerve fascicles, while the echogenic septa correspond to the interfascicular perineurium. Longitudinal sections reveal the fascicular architecture, resembling a “bundle of straws.”[2] Leveraging these characteristics, we used ultrasound to evaluate nerve healing at the distal nerve transfer sites and to observe the biceps muscle’s reaction to electrical stimulation of the donor nerve, thereby confirming muscle re-innervation.
This technical note aims to introduce ultrasound as a simple, non-invasive, and reliable tool for evaluating muscle re-innervation and nerve transfer site healing in distal nerve transfers for brachial plexus injuries. To illustrate its application, we present a case study involving an ulnar nerve fascicle transfer to the biceps muscle in a patient with a C5,6 brachial plexus injury.
TECHNIQUE
We utilize high-frequency linear-array probes (6–15 MHz) for ultrasound imaging, as they offer superior spatial resolution and optimal images with a standard gel interface. Occasionally, low-frequency probes (transducers) are employed for greater tissue penetration depth; however, they are less effective for visualizing superficial structures.[7] The ulnar nerve is visualized and the ulnar fascicle transfer to the biceps branch of the musculocutaneous nerve is visualized. Dynamic imaging is performed during the elbow’s active and passive flexion and extension, primarily to evaluate the biceps muscle. In addition, color Doppler imaging is used to assess vascularity around the nerve healing site, providing valuable information on the status of nerve repair.
ILLUSTRATIVE CASE
A 25-year-old male sustained a C5, C6 brachial plexus injury and developed shoulder and elbow weakness. The surgical intervention included brachial plexus exploration in the neck, spinal accessory nerve transfer to the suprascapular nerve, ulnar nerve fascicle transfer to the biceps branch of the musculocutaneous nerve, and long head of triceps branch transfer to the axillary nerve. The sutures were removed at two weeks. Post-operative electrical stimulation was initiated at six weeks. In addition, passive therapy and shoulder and elbow rehabilitation were started. At three months, ultrasound imaging of the arm confirmed the well-coapted nerve transfer site and demonstrated biceps muscle contractions on cutaneous electrical stimulation of the ulnar nerve. This method could be considered in all nerve transfer surgeries to assess the healing and muscle re-innervation at an earlier rehabilitation stage.
Procedure and findings
At 3 months, an ultrasound of the arm can reveal the nerve transfer site and its healing status. It will show the normal fascicular architecture over the ulnar nerve and its donor nerve fascicle harvested in the proximal arm. By following the donor fascicle, the suture site and the recipient biceps branch of the musculocutaneous nerve can be identified [Figure 1]. Additionally, ultrasound demonstrated biceps muscle contractions in response to cutaneous electrical stimulation of the ulnar nerve [Figure 2] [Video 1]. This non-invasive technique effectively monitored the progress of nerve healing and muscle re-innervation, confirming successful nerve transfer and early muscle recovery.

- The intraoperative picture shows the ulnar fascicle transfer to the biceps branch of the musculocutaneous nerve.
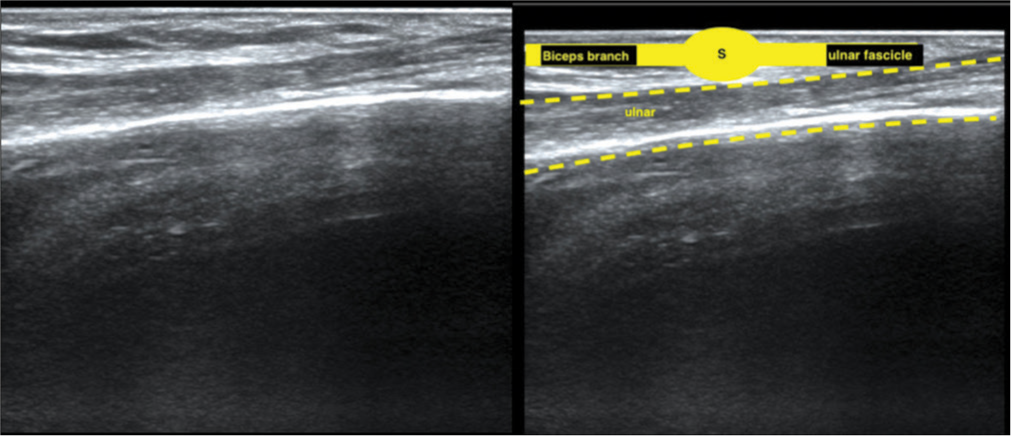
- The ultrasound image of the same patient shows the normal fascicular pattern of the ulnar nerve, ulnar nerve fascicle (yellow), suture site (S), and the recipient nerve biceps branch of the musculocutaneous nerve.
Comparison with other modalities
Clinical recovery, measured by muscle strength using the MRC scale, often takes a long time to become apparent due to the slow process of nerve growth and re-innervation.[6] EMG/NCS studies can detect re-innervation by identifying polyphasic waves and the absence of fibrillations.[3] However, these methods are invasive and can cause patient discomfort. In addition, determining the precise extent and timing of muscle recovery with these techniques can be challenging. In contrast, ultrasound offers a non-invasive, immediate, and patient-friendly alternative for monitoring nerve transfer sites and muscle re-innervation.
Advantages
Ultrasound offers several advantages over traditional methods such as EMG/NCS for assessing nerve transfer and muscle re-innervation. Ultrasound provides real-time feedback, delivering immediate information on nerve transfer site healing and muscle re-innervation. In addition, this method is reproducible and can be reliably used for routine assessments of distal nerve transfer and muscle reinnervation.
Disadvantages
While ultrasound is a valuable tool for assessing nerve transfer healing and muscle re-innervation, it has several limitations. Operator dependency can lead to variability in results, as the accuracy of the assessment heavily relies on the skill and experience of the clinician. Ultrasound also has limited depth penetration, making it challenging to visualize deep structures accurately, especially in patients with a larger body habitus. The resolution may not be sufficient to detect very small or subtle changes in nerve or muscle tissue. In addition, interpreting ultrasound images can be complex and requires extensive training to differentiate between normal and pathological findings. Finally, ultrasound may not provide comprehensive functional information, necessitating complementary use with other diagnostic modalities for a complete assessment.
While our technical note focuses on ultrasound, we acknowledge the need to compare it with EMG in future studies. We hypothesize that ultrasound may offer earlier detection of re-innervation as it can visualize muscle contractions in response to stimulation, potentially preceding detectable EMG changes. However, a prospective study comparing the two modalities is necessary to establish this definitively.
Correlating the ultrasound findings with clinical outcomes is crucial. Unfortunately, due to the nature of this technical note, we focused on the early postoperative period, where clinical recovery may not yet be fully evident. However, in the illustrative case, biceps muscle contractions observed on ultrasound at 3 months were a positive indicator for successful re-innervation.
Impact on management and its use in supercharged endto-side (SETS) transfer
While ultrasound findings alone may not warrant early revision or tendon transfer, they can certainly influence the timing and intensity of rehabilitation efforts. Positive ultrasound findings may encourage more aggressive therapy, while negative findings may prompt further investigation or potential surgical intervention.
We believe that ultrasound could be valuable in assessing SETS transfers, particularly in visualizing the transfer site and monitoring muscle re-innervation.
CONCLUSION
Ultrasound is a valuable tool for diagnosing and monitoring early re-innervation following distal nerve transfers in brachial plexus injuries. It provides a non-invasive, reliable, and patient-friendly method for assessing nerve transfer site healing and muscle contraction in response to donor nerve stimulation. By incorporating ultrasound into the postoperative management protocol, clinicians can enhance the monitoring and outcome of nerve transfer surgeries, ensuring better recovery and function for patients with brachial plexus injuries. We acknowledge that ultrasound is primarily a diagnostic modality. However, providing early evidence of re-innervation and confirming the success of the nerve transfer can guide rehabilitation efforts and potentially optimize functional recovery.
Further research is needed to validate the use of ultrasound in this context and to establish standardized protocols for its application using a prospective study comparing ultrasound with EMG, NCS, and clinical examination in a larger patient cohort.
ETHICAL APPROVAL
Ethical approval was obtained from the Ethical Committee Board of Olympia Hospital and Research Centre, reference number 08/2024, dated June 3, 2024.
DECLARATION OF PATIENT CONSENT
The author certifies that he has obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published, and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY FOR MANUSCRIPT PREPARATION
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
CONFLICTS OF INTEREST
There are no conflicting relationships or activities.
Video is available online at
FINANCIAL SUPPORT AND SPONSORSHIP
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Nerve transfers for traumatic brachial plexus injury: Advantages and problems. J Hand Microsurg. 2011;3:6-10.
- [CrossRef] [Google Scholar]
- Ultrasound cross-sectional area in median nerve axonal loss and demyelination in carpal tunnel syndrome. J Hand Microsurg. 2024;16:100045.
- [CrossRef] [Google Scholar]
- The stages of rehabilitation following motor nerve transfer surgery. J Musculoskelet Surg Res. 2019;3:60-8.
- [CrossRef] [Google Scholar]
- The value of the tender muscle sign in detecting motor recovery after peripheral nerve reconstruction. J Hand Surg Am. 2015;40:433-7.
- [CrossRef] [Google Scholar]
- Rehabilitation of upper extremity nerve injuries using surface EMG biofeedback: Protocols for clinical application. Front Neurosci. 2018;12:906.
- [CrossRef] [Google Scholar]
- Donor activation focused rehabilitation approach: Maximizing outcomes after nerve transfers. Hand Clin. 2016;32:263-77.
- [CrossRef] [Google Scholar]
- Ultrasound as a diagnostic modality in hand and wrist musculoskeletal pathologies: A narrative review. Indian J Orthop 2024
- [CrossRef] [Google Scholar]






