Translate this page into:
Giant cell tumor of the tendon sheath: A critical review of current diagnostic and therapeutic approaches with treatment recommendations for hand and foot lesions
*Corresponding author: J. Terrence Jose Jerome, Department of Orthopaedics, Hand and Reconstructive Microsurgery, Olympia Hospital and Research Centre, Trichy, Tamil Nadu, India. terrencejose@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jerome JTJ, Karunanithi D. Giant cell tumor of the tendon sheath: A critical review of current diagnostic and therapeutic approaches with treatment recommendations for hand and foot lesions. J Musculoskelet Surg Res. 2025;9:28-41. doi: 10.25259/JMSR_246_2024
Abstract
Giant cell tumor of the tendon sheath (GCTTS) is a common tumor affecting the hand and foot, often presenting diagnostic and therapeutic challenges due to its variable clinical presentations and biological behaviors. Recent evidence supports a neoplastic origin for this tumor, previously known by various names. GCTTS can be categorized as localized or diffuse, with distinct predilections for specific anatomical locations. While localized GCTTS is typically benign and more common in the hand and wrist, the diffuse form can be aggressive, also affecting the foot and ankle. This critical analysis review aims to provide a comprehensive overview of GCTTS in hand and foot, encompassing its clinical, radiological, histological, and genomic features. In addition, we summarize current management practices, discuss differential diagnoses, analyze recurrence rates following surgical excision, and offer evidence-based treatment recommendations for this diverse group of tumors.
Keywords
Foot
Giant cell tumor
Hand
Management
Recurrence
Recommendations
Surgical excision
Tendon sheath
INTRODUCTION
Giant cell tumor of the tendon sheath (GCTTS), the second most common hand tumor, is also frequently encountered in the foot and ankle. Despite its prevalence, the exact etiology of GCTTS remains unclear, leading to various names, including pigmented villonodular synovitis and fibrous xanthoma. Several theories, such as neoplastic, inflammatory, and trauma-induced origins, have been proposed. However, recent evidence from DNA analysis strongly supports a neoplastic origin for GCTTS.[1-4]
Historically categorized as localized (nodular) or diffuse, GCTTS is now recognized as tenosynovial giant cell tumor (TSGCT), encompassing lesions affecting synovial membranes of joints, bursae, and tendon sheaths.[1-5] GCTTS can be classified as localized or diffuse based on clinical presentation and biological behavior. Localized (nodular) GCTTS, typically benign, commonly occurs in the digits and wrist,[1-6] while the diffuse type, often exhibiting aggressive behavior, is more prevalent in larger joints like the ankle and knee.[1,3,5,7-12]
The diverse clinical presentations, anatomical locations, and biological behaviors of GCTTS, particularly in the hand and foot, pose challenges in management.[7-30] This review aimed to elucidate the key clinical, radiological, histological, and genomic features of GCTTS in these regions. Furthermore, we summarize current management practices, explore differential diagnoses, analyze recurrence rates following surgical excision, and critically evaluate the existing literature to provide evidence-based treatment recommendations for this heterogeneous group of tumors.
CLINICAL PRESENTATION AND EPIDEMIOLOGY
The reported incidence of localized GCTTS ranges from 30 to 34 cases/million person-years for those affecting digits and 11 cases/million person-years for those located in the extremities. Meanwhile, diffuse-type incidence is estimated to be between 5 and 8.4 cases/million person-years.[1-6,30-38] It predominantly affects younger populations, with the localized form typically appearing in the fourth and fifth decades of life, while the diffuse form often manifests slightly earlier, usually before age 40. While rare in children, GCTTS can occur across all age groups.[7-15]
There is a notable sex disparity in GCTTS prevalence, with the localized nodular form exhibiting a 2:1 female-to-male ratio.[1,3,5,7,9,27] Conversely, the diffuse form demonstrates only a slight female predominance. Clinically, GCTTS presents with non-specific symptoms, making it a diagnosis of exclusion when other synovial pathologies have been ruled out.
Localized giant cell tumors (GCTs) are most commonly found in the fingers, representing 85% of cases, and typically occur near synovial sheaths or interphalangeal joints, usually on the palmar side. GCTs can also develop less frequently in the wrist, foot, ankle, knee, hip, and elbow. Among joints with intra-articular involvement, the knee is the most frequently affected.[1,2,4,6,8-12,27]
Although any synovial joint can be impacted, including the temporomandibular and spinal inter-apophyseal joints, these tumors are typically confined to a single joint. Rarely do multifocal instances occur, often showing bilateral involvement of the same joint (knee or ankle) or appearing as multifocal forms, particularly in children.[1,2,4,6,8-12,27]
Extra-synovial soft-tissue forms of GCTTS predominantly affect the knee, thigh, or foot, typically arising in periarticular tissue, with intramuscular and subcutaneous forms also observed.[2]
In approximately half of the cases, patients report a history of trauma, although the causal relationship remains unclear. The progression of symptoms is typically gradual, with intervals between initial presentation and diagnosis ranging from 10 months to 3 years.[1,3,5,7,9,27]
FUNCTIONAL SYMPTOMS
Extra-articular and tendon sheath forms usually manifest as a slowly growing, painless mass that can cause skin tension in the fingers or toes, potentially leading to discomfort when wearing shoes[2,4,6,27]
Articular forms often lead to discomfort, recurrent swelling, and limited range of motion. In the knee, symptoms may mimic meniscal or instability.[2,4,6,27]
CLINICAL EXAMINATION FINDINGS
During a clinical examination, a soft, palpable mass is often detected in superficial locations, occasionally accompanied by warmth, periarticular effusion, or edematous swelling. Although the clinical findings may lack specificity, they can be suggestive in certain cases, particularly in young adults or when the mass is located in typical areas such as the soft tissue of the hand or foot.[2,4,6,27]
On the hand, GCTTS typically presents as a slow-growing mass on a finger and less commonly on a metacarpal or wrist [Figure 1]. Most tumors are asymptomatic, but some patients may experience pain, numbness, or reduced finger mobility. The primary nodule may be accompanied by benign satellite nodules, which pose no risk of malignant transformation regardless of their growth rate or size. Symptoms before diagnosis usually persist for 2–12 months, with an average duration of 6.1 months.[2,4,6,11,27]
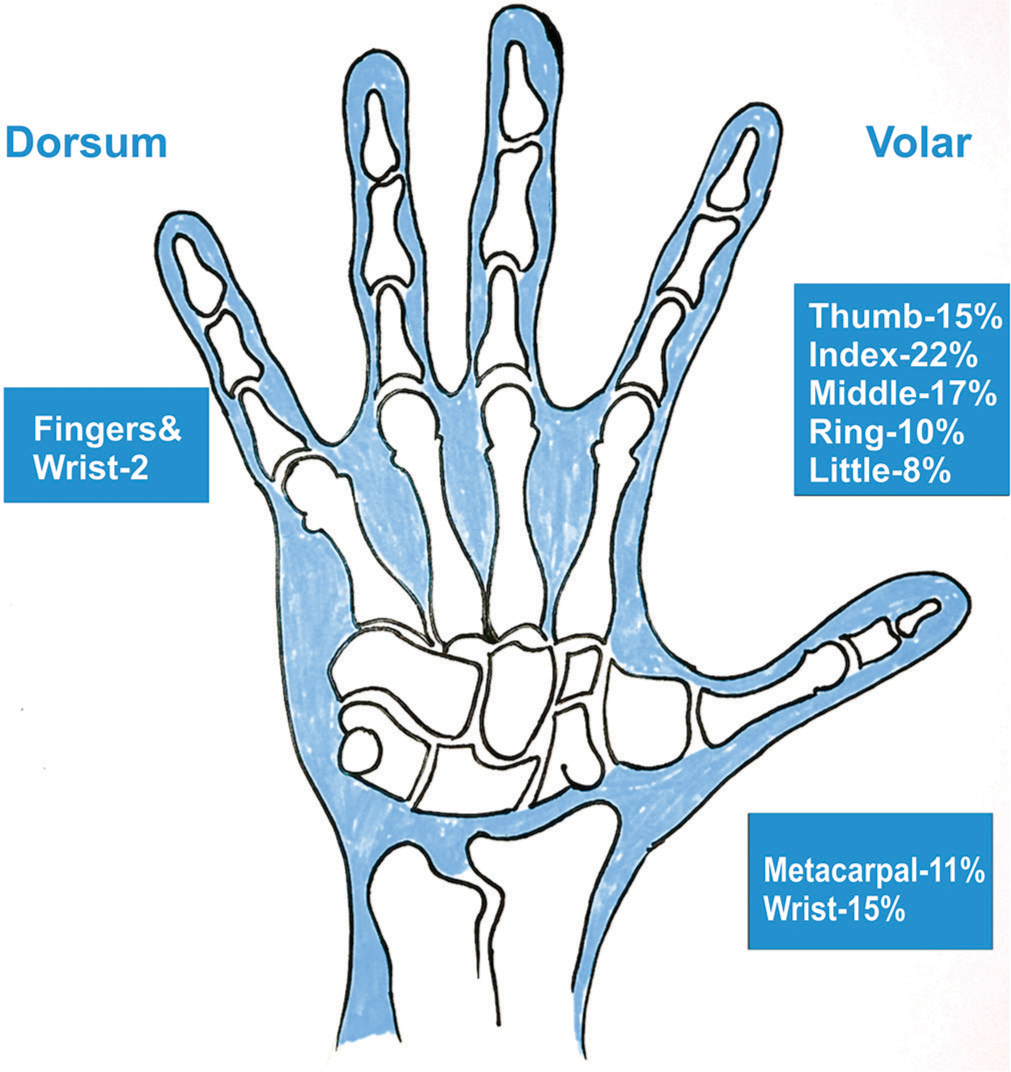
- Illustrative image showing the common locations of the tumors in the hand.
Patients with GCTTS in the foot and ankle [Figure 2] usually present with various common complaints and clinical features. Most report a painless, palpable mass in the affected area, which is typically solitary, solid, and well-defined. Some experience discomfort when bearing weight, particularly if the mass is located on the plantar aspect of the foot.[10,11,27] The masses may exhibit varying degrees of mobility on physical examination. In general, patients do not report difficulty with footwear or walking limitations, although some discomfort may be noted. In cases where the mass compresses nearby nerves or joints, patients may experience limitations in joint movement, such as plantar flexion or sensory deficits.[10,11,27] A subset of patients may have a history of trauma that precedes the appearance of the mass. Symptoms typically persist for an extended period before presentation, with an average duration of 3.5 years, ranging from 1 month to 20 years. These clinical features help guide the evaluation and management of GCTTS in the foot and ankle.
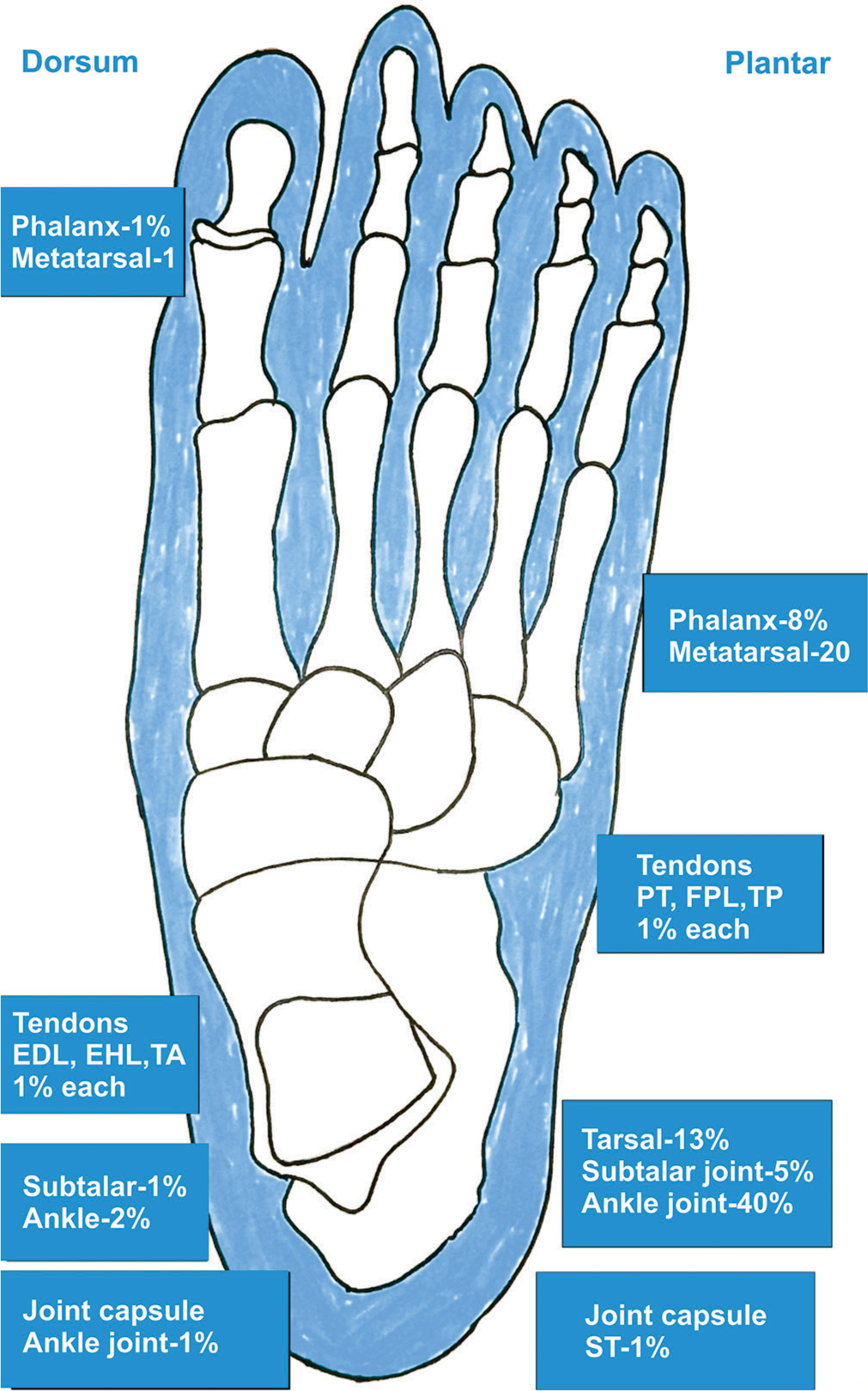
- Illustrative image showing the common locations of the tumors in the foot and ankle. EDL: Extensor digitorum longus, EHL: Extensor hallucis longus, TA: Tibialis anterior, TP: Tibialis posterior, FPL: Flexor pollicis longus, ST: Semitendinosus tendon, PT: Pronator teres.
WORKUP AND DIAGNOSIS
Imaging studies
The diagnosis of TSGCT relies heavily on clinical examination and imaging. Radiographs may show a non-specific soft-tissue mass or peripheral calcification. Osseous erosion or invasion is rare.[2,4,6,11,27] However, when the tumor compresses the phalanx, it can create an impression on the cortex visible on a radiograph [Figures 3 and 4].
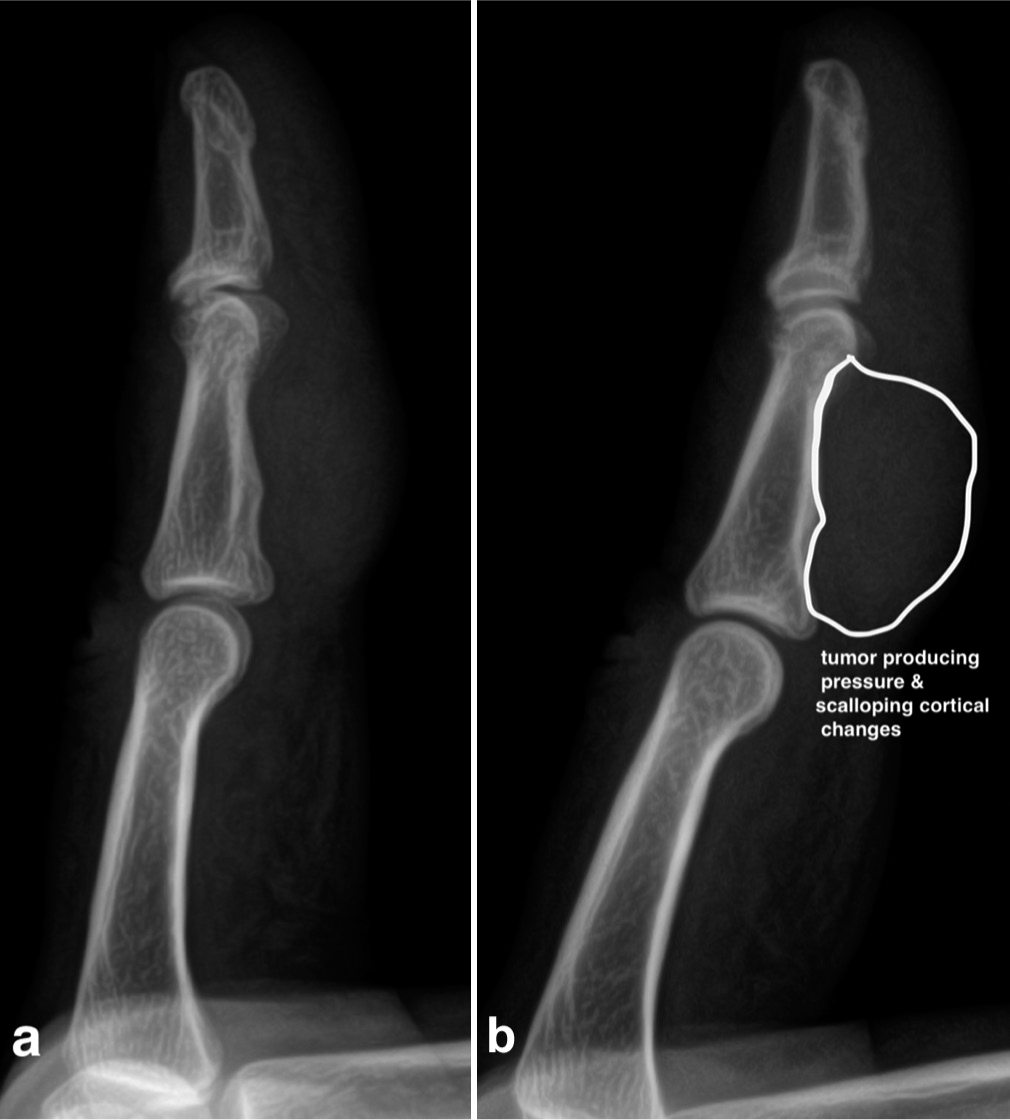
- (a, b) The radiograph of the finger shows a large tumor exerting pressure (indentation) on the surrounding cortex, indicating cortical erosion due to its invasive and progressive nature.
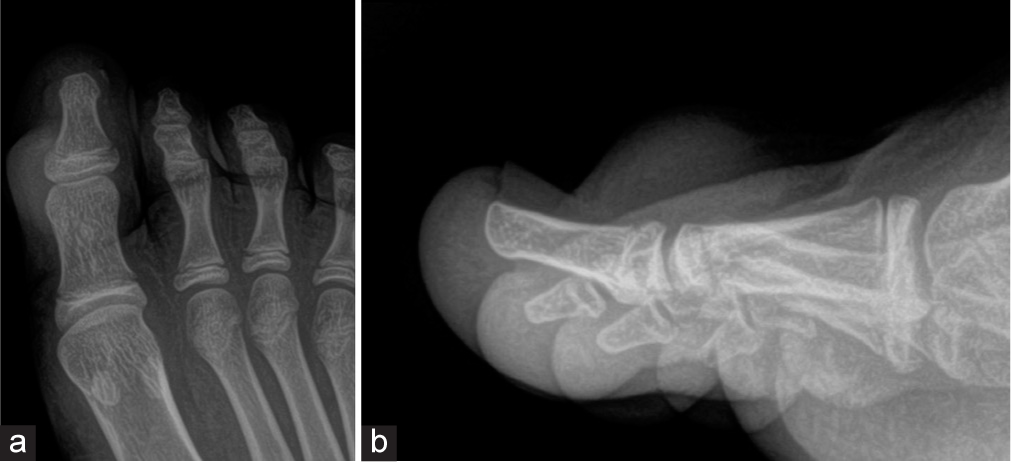
- The radiograph of the foot shows (a, b) a soft-tissue lesion on the dorsum of the great toe without cortical involvement.
Ultrasonography can confirm the presence of a lesion, revealing either homogeneous hypoechoic areas or heterogeneous echoic areas. It can identify a solid hyperplastic lesion within the finger [Figure 5]. In addition, a color Doppler examination may show increased vascularity. Computed tomography typically reveals a solid, heterogeneous, and hypodense mass with heterogeneous enhancement on contrast-enhanced scans [Figure 6].[2,4,6,10,11,27]
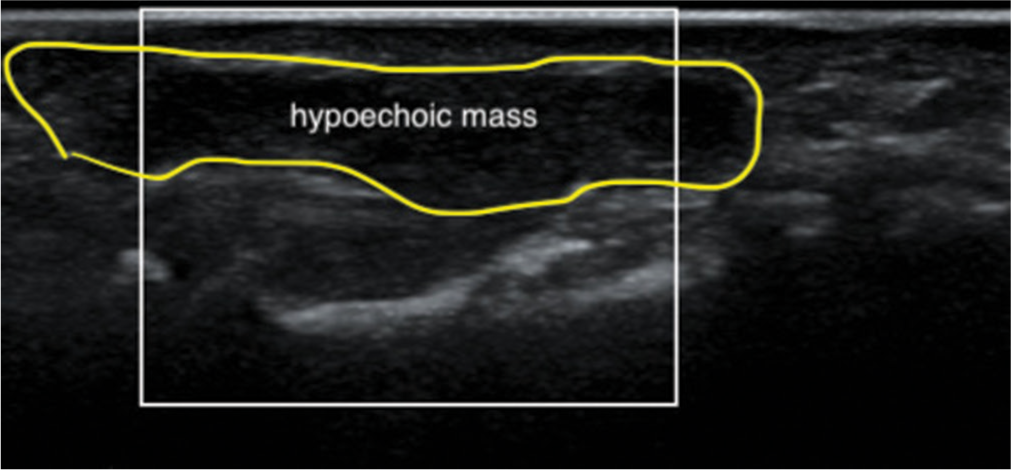
- Ultrasound images of the finger at the middle phalanx level show a solid, homogeneous, hypoechoic mass immediately adjacent to the flexor tendons, suggesting Giant cell tumor of the tendon sheath.
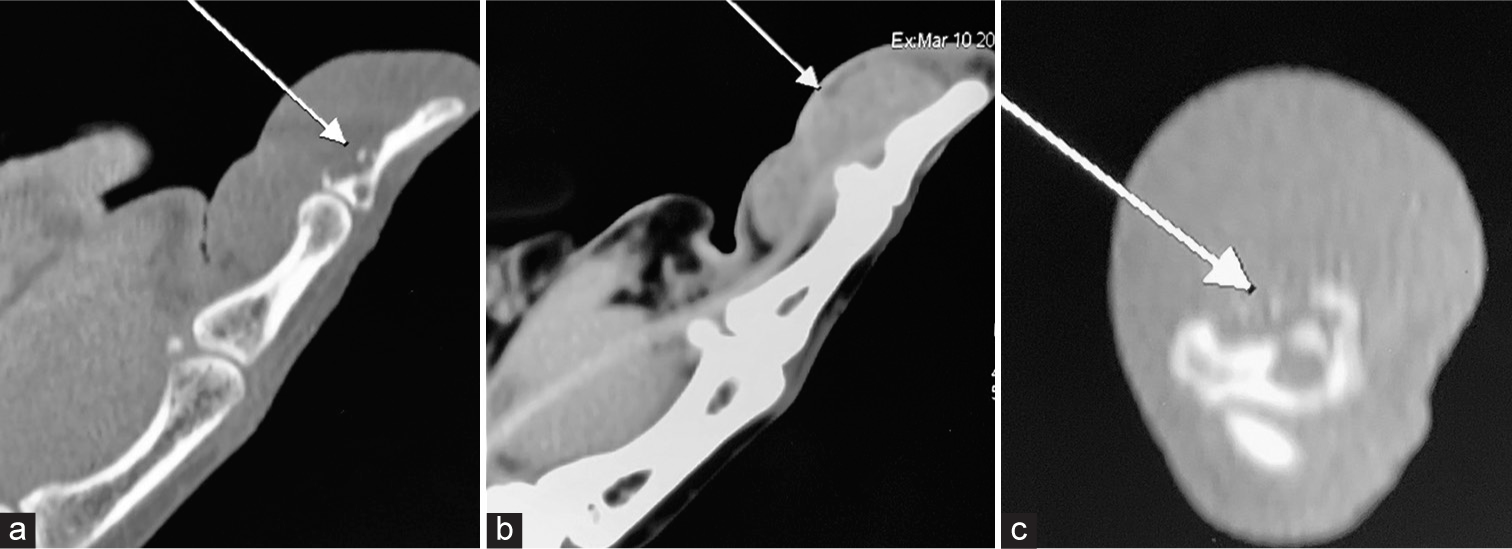
- Computed tomography (CT) of the patient involving the interphalangeal joint of the thumb shows (a) osteolytic erosion in the distal phalanx near the flexor pollicis longus tendon attachment (white arrow), (b) solid hypodense mass with homogeneous enhancement on contrast-enhanced CT (white arrow), and (c) intra-osseous invasion in axial views (white arrow).
Magnetic resonance imaging (MRI)
MRI is a critical tool in the diagnosis and surgical planning of GCTTS. MRI typically reveals a well-defined lesion in localized forms, often situated adjacent to or partially encircling the affected tendon. Diffuse forms are commonly intra-articular, presenting as a soft-tissue mass with uniform enhancement and associated joint effusion.[2,4,6,10,11,27] Pre-operative MRI assessment should also evaluate for possible involvement of the fossa or extra-articular extension. The signal intensity on MRI can differentiate between localized and diffuse GCTTS. The varying levels of hemosiderin deposition within the lesion result in variable signal intensity on T1 and spin-echo T2-weighted sequences. The signal is typically enhanced with gadolinium injection. Gradient-echo sequences are particularly valuable in detecting hemosiderin deposits, which appear as low-signal areas, even after contrast administration.
MRI plays a crucial role in diagnosing GCTTS [Figure 7].[2,4,6,10,11,27] The varying signal intensity on T1- and T2-weighted images, particularly when enhanced with gadolinium, allows for differentiation between localized and diffuse forms of GCTTS, guiding subsequent treatment decisions. Using gradient-echo sequences, the modality’s ability to detect hemosiderin deposits, a hallmark of GCTTS, further solidifies its diagnostic value. In addition, MRI aids in assessing the extent of tumor involvement, including extra-articular extension, which is crucial for surgical planning. MRI also facilitates the differentiation of GCTTS from other similar-appearing lesions and can identify associated features like joint effusion and bone erosion, thus providing a comprehensive assessment of the tumor and its impact on surrounding tissues.
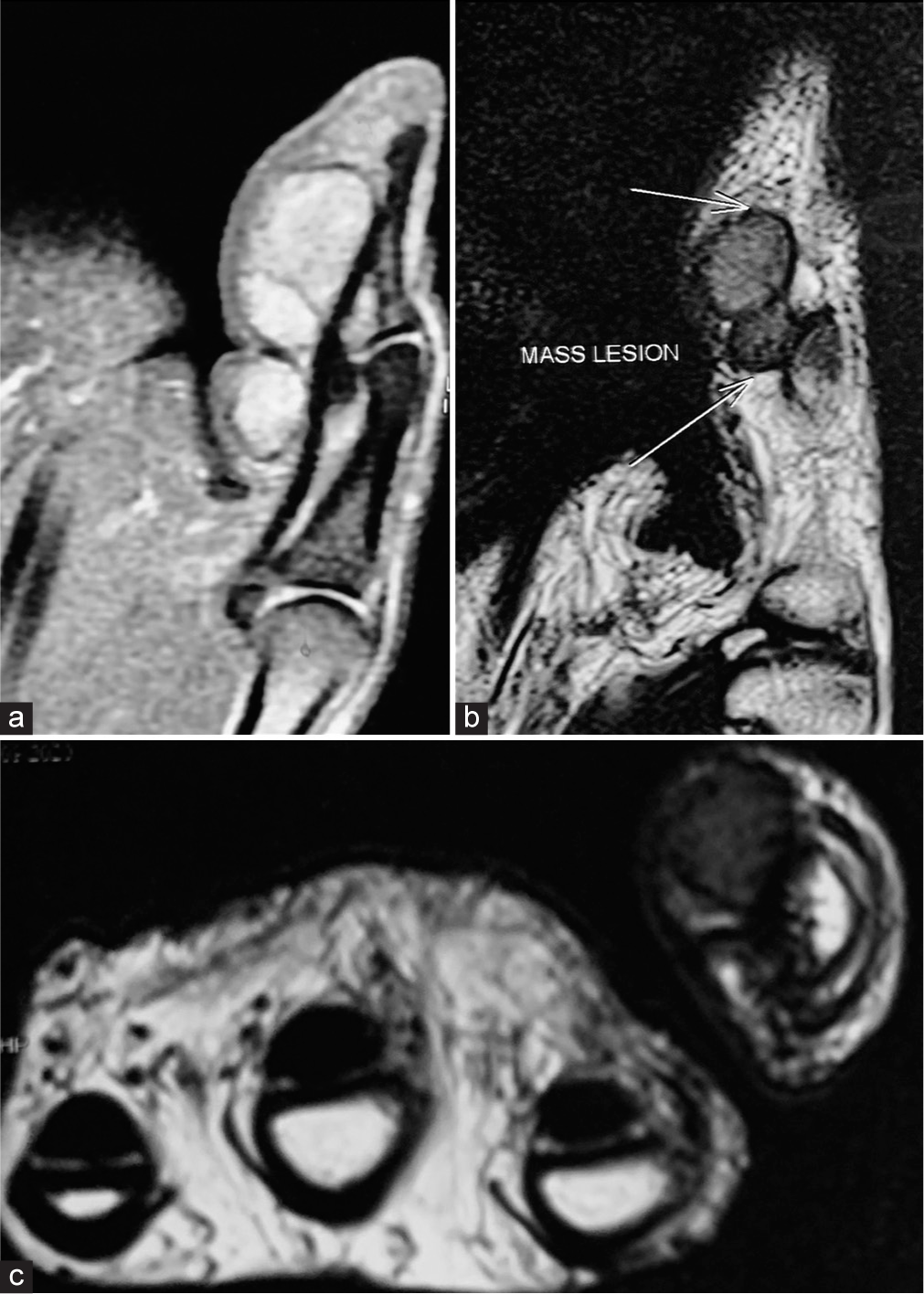
- Sagittal (a and b) T1- and T2-weighted and axial (c) magnetic resonance imaging images demonstrate a large, lobulated soft-tissue mass occupying the volar aspect of the distal phalanx and interphalangeal joint, encasing the flexor pollicis longus tendon. In addition, the images reveal intraosseous invasion at the base of the distal phalanx.
Role of fine need aspiration cytology (FNAC)/Biopsy
Given its minimally invasive nature and ability to provide rapid preliminary results, FNAC can be a valuable initial diagnostic tool in suspected cases of GCTTS. However, results should be interpreted cautiously due to potential sampling errors and overlapping cytological features with other entities. A definitive diagnosis should always be confirmed through histopathological examination of a larger tissue sample obtained through open biopsy or excision.[33] In addition, incorporating MRI imaging can offer crucial information regarding tumor characteristics and aid surgical planning. In cases of diagnostic uncertainty or atypical features, molecular analysis of a biopsy sample can further differentiate GCTTS and provide insights into its biological behavior, ultimately guiding personalized treatment decisions.
The diagnosis could sometimes remain uncertain even after imaging studies. However, in cases where GCTTS is suspected in the hand or foot, a biopsy may not be necessary if the lesion can be completely excised during a single surgical procedure. Complete excision often serves as both a diagnostic and therapeutic approach, making a separate biopsy unnecessary. Some researchers have proposed that chromosomal analysis of biopsy specimens could help determine the appropriate extent of surgical intervention.[27] However, these protocols have not yet gained widespread acceptance as standard practice.
In contrast, for GCTTS located in less accessible areas such as the thigh, knee, or forearm, a tru-cut biopsy might be considered to establish a clear diagnosis before planning the definitive treatment.[27] However, this approach is less common in areas like the ankle, where only limited attempts have been documented. The risk of contaminating the surgical field and potentially compromising patient outcomes is a concern during any biopsy procedure, and this is particularly critical in anatomically complex regions.
While it might be tempting to do a biopsy of the extremity lesions, incomplete excision of a potential tumor can result in recurrence and significant morbidity. Incomplete removal may lead to surgical field contamination, making subsequent surgeries more challenging and possibly converting a salvageable situation into one that is not. When the tendon or joint capsule is directly involved, surgeons should adopt a more aggressive approach and monitor these patients closely for recurrence rather than doing a biopsy alone. These patients need definitive and complete surgical removal.
Therefore, these cases should be carefully evaluated and discussed in multidisciplinary team meetings to decide on the best approach – whether to proceed with a biopsy or to opt for complete surgical excision. More research is needed to establish guidelines and recommendations, particularly for biopsies in less common locations such as the hand and ankle, to ensure the most effective and safe management of GCTTS.
PATHOLOGY
Al-Qattan[4] classified the macroscopic appearance of the hand into two main types based on the presence or absence of a single, encircling pseudocapsule during surgical assessment. Type I tumors consist of a single, well-defined mass, which can be either round or multi-lobulated. In contrast, Type II tumors present as two or more distinct, separate masses that are not interconnected [Tables 1, 2 and 3]. This classification system aids in surgical planning and predicting tumor behavior.
| Type 1 | Type 2 |
|---|---|
| The entire nodule is surrounded by one pseudo-capsule | The entire nodule is not surrounded by one pseudo-capsule |
|
|
| Study | Recurrence rate (%) |
|---|---|
| Byers et al. (1968) | 27 |
| Grover et al. (1998)[42] | 15 |
| Jones et al. (1969) | 18 |
| Looi et al. (1999)[43] | 7 |
| Rao and Vigorita (1984)[44] | 29 |
| Al-Qattan (2001) | 12 |
| Ozalp et al. (2004)[45] | 14 |
| Lowyck and De Smet (2006) | 16 |
| Williams et al. (2010) | 14 |
| Di Grazia et al. (2013) | 5 |
| Ozben and Coskun (2019) | 6 |
| Zyluk et al. (2020)[6] | 21 |
| Benign |
| Tenosynovial giant cell tumor |
| Deep benign fibrous histiocytoma |
| Intermediate (rarely metastasizing) |
| Plexiform fibrohistiocytic tumor |
| Giant cell tumor of soft parts NOS |
| Malignant |
| Malignant tenosynovial giant cell tumor |
NOS: Not otherwise specified.
Grossly, GCTTS appears as a well-circumscribed, mostly solid, multinodular mass with a fleshy, red-brown, or gray cut surface.[4,6,11,27,39-41] It is well-encapsulated and ranges from 1.5 cm to 5 cm in diameter. Its color varies from tan and yellow to brown and dark red, often due to prominent hemosiderin deposition.
HISTOPATHOLOGY
The microscopic examination reveals classic features in all cases.[4,6,11,27,39-41] These features included the presence of numerous:
-
Multinucleated giant cells: These are large cells containing multiple nuclei, a hallmark of GCTs [Figure 8].
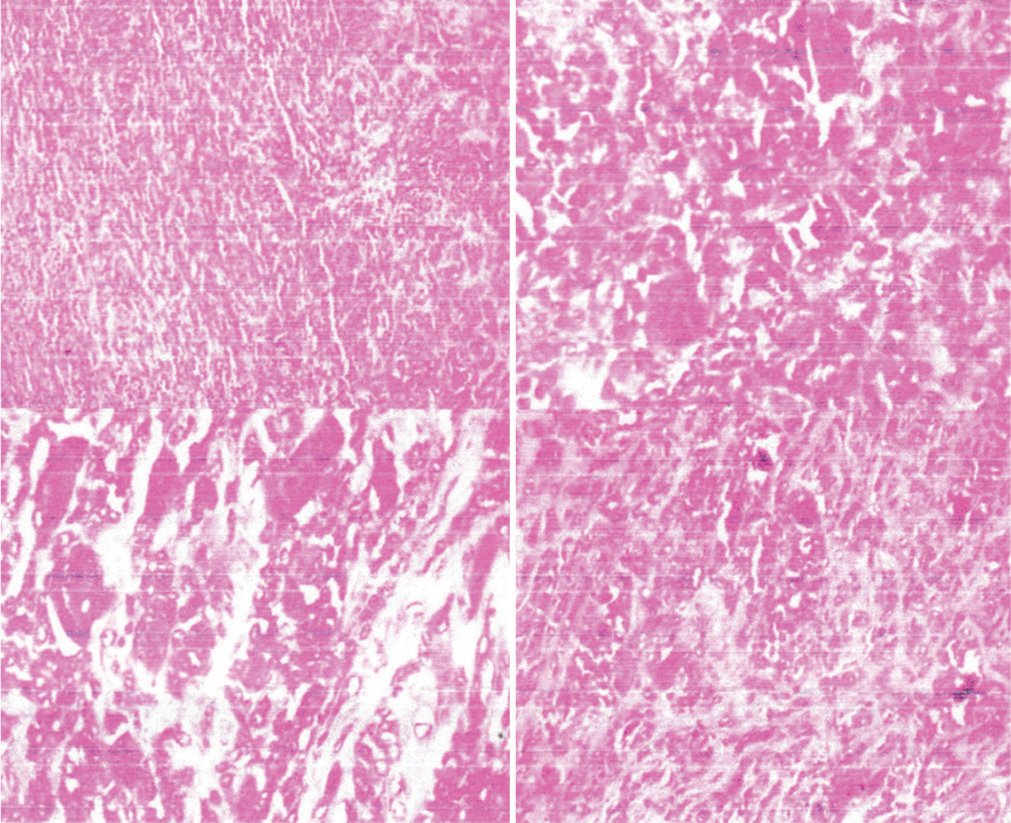 Figure 8:
Figure 8:- The histopathological sections reveal a circumscribed proliferation of benign tenosynovial cells, along with collagen deposition, small giant cells, and foamy macrophages. These findings are consistent with a benign giant cell tumor of the tendon sheath. Notably, there are no histological features indicative of neurofibroma, lipoma, or malignancy.
Round or polygonal mononuclear cells: These are single-nucleus cells with varying shapes, another characteristic component of the tumor.
Foam cells or histiocytes: These are cells that contain fat droplets. They are often seen in GCTTS and contribute to its pathology.
Synovial remnants, characterized by a villous lobular architecture, exhibit cellular heterogeneity both between different tumors and within a single tumor. Histiocytes, which stain positive for CD68, are the predominant cell type, accompanied by varying proportions of foamy cells, chronic inflammatory cells, and multinucleated giant cells. The mitotic rate can fluctuate, but atypical cells are not typically observed.[4,6,11,27] However, Bertoni et al. have documented rare instances of malignant transformation in these lesions.[5]
PROGRESSION OF THE GCTTS
The progressive potential of GCTTS varies depending on the location and type of lesion. Understanding this progression is essential for assessing treatment options.
Hand and wrist
Extra-articular forms in the hand and wrist usually behave as slowly progressing masses (12 months–3 years). The lesion is confined within the anatomically narrow spaces and can grow along the tendon sheaths, sometimes causing bone notches. However, joint deterioration is less frequent in the hand and wrist compared to larger joints.[2,4,6,12-15]
Foot and ankle
In the foot and ankle, diffuse GCTTS can lead to joint deterioration (chondrolysis) and intra-osseous extension, similar to larger joints such as the knee and hip. The risk of progression to osteoarthritis (OA) in the hindfoot is less clear due to limited data.[2,9-11,15,27,31-33] Some cases have required talocrural fusion, while others have shown stabilization without intervention.
VARIABILITY IN PROGRESSION
GCTTS progression is highly variable and not always predictable. Some cases remain stable without treatment or after incomplete synovectomy, while others exhibit renewed growth. Malignant transformation is exceptionally rare, with few documented cases in the literature.
While the risk of malignant transformation is minimal, the primary concern with GCTTS progression is the potential for chondrolysis and subsequent OA, particularly in the ankle.[2,9-11,15,16,27] The exact roles of the disease itself and surgical intervention in this progression remain to be fully elucidated.
MALIGNANT GCTTS
Malignant GCTTS is a rare but aggressive sarcoma with the potential for local destruction, metastasis, and mortality.[5,38,40,41] It can either develop spontaneously or arise from a pre-existing benign GCTTS following multiple recurrences. This transformation is often challenging to diagnose due to the subtle histological changes that may occur. Clinically, malignant GCTTS often presents as a large, rapidly growing, and painful mass near major joints.[5,38,40,41] Imaging studies, such as MRI, may reveal an expansile or infiltrative lesion with heterogeneous signal intensity, reflecting the variable composition of the tumor.
Histologically, malignant GCTTS exhibits a complex and diverse morphology, with areas of benign GCTTS interspersed with frankly malignant components. These malignant areas may resemble various sarcoma types, including malignant fibrous histiocytoma, fibrosarcoma, or GCT. The malignant cells typically demonstrate increased cellularity, nuclear atypia, mitotic activity, and necrosis.[5,38,40,41] Immunohistochemical markers such as clusterin and RANKL can aid in distinguishing malignant from benign components.
Despite recent advances in targeted therapies, such as CSF1R inhibitors, the prognosis for malignant GCTTS remains poor, with high rates of local recurrence and distant metastasis, particularly to the lungs and lymph nodes. Conventional cytotoxic chemotherapy, particularly with doxorubicin-based regimens, has shown some clinical benefit in select cases.[5,38,40,41] Due to the rarity of malignant GCTTS, further research is needed to understand its pathogenesis better, refine diagnostic criteria, and develop more effective treatment strategies. A prospective registry of patients with malignant GCTTS could facilitate collaborative research and improve our knowledge of this challenging disease.
TREATMENT STRATEGIES
Although rare case reports of malignant transformation or aggressive progression exist, these occurrences are insufficiently documented to significantly influence therapeutic decision-making for GCTTS, which is generally considered a benign process.
Localized GCTTS
Localized GCTTS typically allows for complete surgical resection, resulting in low recurrence rates (0–15%) regardless of intra- or extra-articular location.[2,4,6,10,11,16,27] Most patients (73–91%) experience no local recurrence at five years, with excellent or good clinical outcomes reported after surgical treatment.
Diffuse GCTTS
Diffuse GCTTS poses surgical challenges, leading to higher recurrence rates (21–50%). While 27–62% of patients remain recurrence-free at five years, several factors contribute to the increased risk.[2,4,6,10,11,16,27]
Diffuse form
This is a consistent risk factor across all studies.
Incomplete synovectomy
This has been associated with higher recurrence rates (44– 55%) than total resection (0–9%).
Other potential factors such as bone erosion, cyst formation, tumor size >2 cm, and tendon or neurovascular involvement, have been suggested as risk factors, but with less conclusive evidence.
SURGICAL APPROACH
Extensive en bloc resection, typically used for malignant tumors, is not indicated for GCTTS.[2,4,6,10,11,16,27,31-33] Instead, resection can be total or subtotal, depending on various factors such as disease history, clinical presentation, tumor type, extent, location, and progression. While surgical excision is the primary treatment for GCTTS, recurrence rates vary significantly between localized and diffuse forms. Complete resection is crucial for minimizing recurrence, particularly in diffuse cases.
FOREFOOT
In the forefoot, GCTTS lesions are primarily localized and managed similarly to those in the hand. Maximal resection of pathological tissue through open surgery is recommended for symptomatic and progressive cases.[10,11,27]
HINDFOOT
Hindfoot lesions are typically articular, affecting the talocrural or subtalar joints, often with bone and extra-articular involvement. Surgical intervention should prioritize minimizing iatrogenic risk, as spontaneous regression has been observed in some cases after partial surgery. Arthroscopy is preferred for accessible lesions, demonstrating successful treatment without recurrence in limited reports.[10,11,27] However, open surgery is necessary for diffuse forms, especially with extra-articular involvement, and fusion may be required in cases of extensive cartilage or bone damage.[10,11,27]
HAND
In the hand, 78% of lesions are nodular, with multiple, independent nodules being rare.[12] Although hand tumors usually present with mild symptoms, the mass can cause discomfort or be aesthetically unappealing. Surgical removal carries a 14.8% risk of local recurrence, which is higher in cases with multinodular forms.[12] To minimize recurrence rates, maximal resection using magnification or a microscope is essential (5%).
RADIOTHERAPY
Radiation therapy (RT), the most commonly employed adjuvant treatment, can potentially reduce recurrence in diffuse GCTTS, particularly after incomplete synovectomy, although evidence supporting its use remains limited.[16-25] External beam RT, utilizing 30–50 Gy in 20 fractions, offers high disease control rates (95.1%) in cases of partial resection or extensive local recurrence, with minimal impact on function.[18] However, its application in this benign condition is infrequent.
Jerome et al.[23] administered a total radiation dose of 21 Gy to the tumor over 14 sessions within three weeks using the three-dimensional conformal RT (3DCRT) technique. This approach effectively focused the radiation on the target area while protecting the surrounding normal tissues, successfully preventing recurrence. Despite this, Jones et al.[34] reported that three out of four patients experienced a recurrence even with adjuvant RT. In contrast, Kotwal et al.[17] observed no recurrences in 14 patients who underwent both excision and RT, with a mean follow-up period of 52 months. Vardakas et al.[35] reported a single case successfully treated with radiation following multiple excisions. In addition, O’Sullivan et al.[21] documented 14 cases of diffuse-pigmented villonodular synovitis treated with RT, with only one recurrence. Intra-articular RT (isotopic synoviorthesis) is not recommended when there is bone involvement or if arthroplasty is planned. While theoretically associated with a risk of malignant transformation, no such cases have been documented following RT for GCTTS. Overall, the available literature suggests potential benefits of RT, particularly external beam RT, warranting further clinical studies to establish its definitive role.
CHALLENGES IN HAND MANAGEMENT
Surgical excision
Surgical removal is the primary treatment for GCTTS [Figure 9]. The goal is to excise the tumor completely with a clear margin to reduce the likelihood of recurrence. Incomplete excision is the most significant factor contributing to tumor recurrence.[2,4,6,12,14,15,23,26] Using magnification tools, such as surgical loupes or operating microscopes, can help surgeons differentiate between healthy and diseased tissues, ensuring more precise excision.
Pre-operative evaluation
Pre-operative assessment includes a thorough physical examination and imaging studies, such as ultrasound or MRI, to understand the tumor’s relationship with surrounding structures. Ultrasound can confirm the presence of a solid hyperplastic lesion within the finger, while MRI provides detailed information on the tumor’s relationship with adjacent joints, nerves, and blood vessels. However, these imaging techniques are not always necessary unless the tumor’s location and size suggest potential complications.
Post-operative care
Post-operative care involves monitoring for signs of recurrence. Follow-up examinations are crucial and should continue for several years post-surgery, as recurrences often occur within the first two years. Patients are advised to report any new symptoms or changes in the operated area immediately.
Factors affecting recurrence rate
The post-operative recurrences reported in the literature range from 4% to 27%.[2,4,6,12,14,15,23,26] The most significant factor influencing recurrence is incomplete tumor excision [Table 4]. Only some lesions are surrounded by a well-formed connective tissue capsule, which facilitates enucleation of the entire tumor. In tumors without a capsule, distinguishing healthy tissue from diseased tissue is challenging, often resulting in residual tumor fragments within the incision, leading to recurrence. The presence of satellite nodules also promotes relapse by being easily overlooked during the removal of the primary lesion [Table 5].
Other contributing factors reported in the literature include various surgical and patient-related aspects. However, the actual significance of these factors remains uncertain due to varied post-operative follow-up periods, ranging from 6 months to 10 years. Publications with less than a year follow-up periods generally report lower recurrence rates than those with longer-term results. Therefore, the optimal time for assessing recurrence after surgery should be at least three years. While distant metastases are rare, they primarily occur in the lungs. Long-term follow-up is essential to monitor for local recurrence and metastasis.
Review of surgical treatment outcomes
Williams et al.[13] reported on 186 patients with GCTTS, finding a recurrence rate of 14% over a 5-year follow-up.
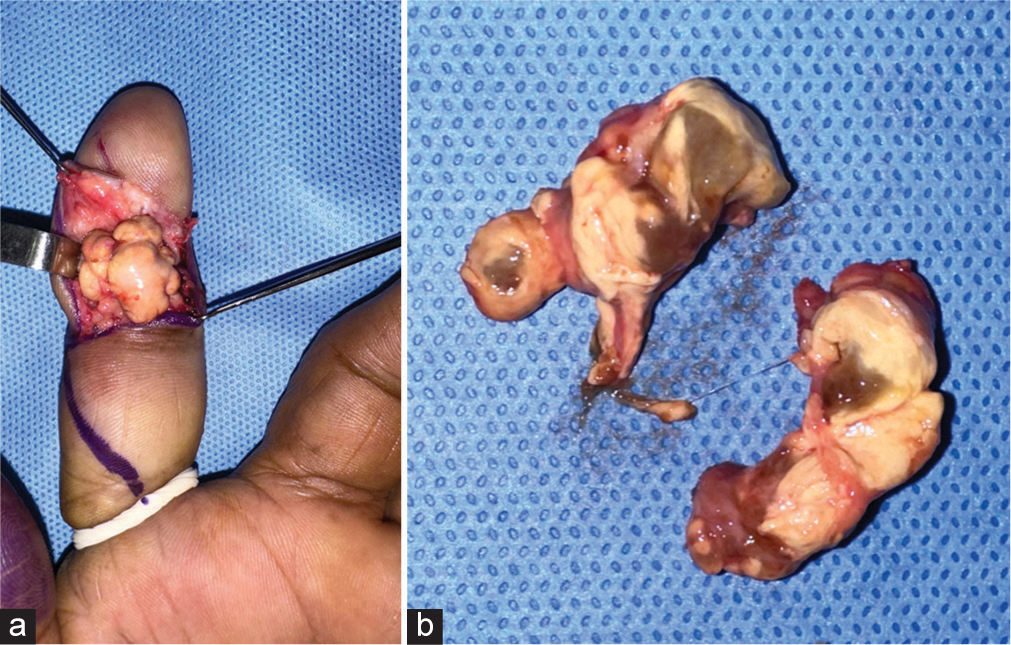
- (a) Intraoperative picture of the large tumor in the volar aspect of the index finger. (b) The tumor is pedunculated, and the cut section shows multilobular, yellowish-brown due to the hemosiderin infiltration.
| Poor surgical technique/incomplete excision (leaves micro disease behind) |
| Bone invasion by the tumor (pressure erosion of the bones and joints on radiographs) |
| Location near the interphalangeal joint |
| Location near the neurovascular structures in fingers |
| Degenerative changes in the joint near the tumor in radiographs |
| Cellularity and mitotic activity on histological examination |
| Tumor with nm 23 negative |
| Type II tumors |
| Histological atypia |
| Tumors involving joint capsule and the flexor tendon in hand |
Factors influencing recurrence included tumor involvement of the tendon or joint capsule, with a 32% recurrence rate in such cases compared to 4% when these tissues were not involved. Post-operative complications occurred in 25% of patients, mostly transient sensory disturbances.
Di Grazia et al.[14] reviewed 64 patients and noted a 5% recurrence rate. Operations performed under magnification reported fewer recurrences. Tumor location near the neurovascular bundle or tendons did not influence recurrence rates significantly. Ozben and Coskun[15] reported a 6% recurrence rate in 50 patients over a 7-year follow-up. Tumors located near interphalangeal joints had a higher risk of recurrence. Fotiadis et al.[12] conducted a meta-analysis of 21 publications covering 605 patients over 30 years. The recurrence rates varied from 0% to 50%, with radical excision using magnifying optical devices being the most crucial factor in reducing recurrence. Type 1 tumors in the Al-Qattan classification[4] had significantly lower recurrence rates compared to type II tumors. While surgery is the primary treatment for GCTTS, radiotherapy has been explored to reduce recurrence rates. One study reported only a 4% recurrence rate after using RT as adjuvant therapy. However, subsequent publications have not conclusively confirmed the benefits of RT, and it is generally not used due to potential harm and costs.
The outcomes of treating the hand indicate that it is a moderately common condition in surgical practice but is associated with a relatively high recurrence rate. This high recurrence rate is primarily attributed to inadequate surgical excision, often due to excising nodules without sufficient margins, sometimes in fragments, and performing surgeries without the aid of magnifying devices. These factors increase the risk of incomplete excision, especially when the tumor lacks a well-developed capsule or is accompanied by satellite nodules.
The successful management of GCTTS in the hand hinges on meticulous surgical technique, including the use of magnification to ensure complete tumor excision. Identifying tendon and joint capsule involvement during surgery can help hand surgeons categorize patients into high-risk and low-risk groups [Table 6].[39] When the tendon or joint capsule is directly involved, surgeons should adopt a more aggressive approach and monitor these patients closely. Conversely, without such involvement, a less extensive excision may be sufficient. This stratification can also enable researchers to concentrate on adjuvant therapies for high-risk patients, sparing low-risk patients from unnecessary morbidity and costs. Additional research is needed to establish the optimal management strategies for high-risk patients. Ensuring precise and complete excision during the initial surgery remains the most effective strategy to minimize the risk of recurrence.
CHALLENGES IN FOOT MANAGEMENT
Location
Zhang et al.[27] studied 20 patients, consisting of 14 females and six males, with an average age of 38.7 years, ranging from 15 to 59 years. Darwish and Haddad’s research on 52 patients demonstrated that GCTTS can occur at any age, with their patients’ ages ranging from 6 to 65 years and peak incidences in the 20–29 and 40–49 age groups. Similarly, Zhang et al. found that GCTTS in the foot and ankle affected individuals between 15 and 59 years, with a higher prevalence among females.[27] Although GCTTS is rare in children, Occhipinti et al. reported cases in a 6-year-old girl and a 12-year-old boy, both presenting with tumors in their toes.[31]
In the study by Zhang et al.,[27] the lesions typically developed in the forefoot and hindfoot, especially around the second toe. Tumors can also originate from various tendon sheaths, including those of the tibialis posterior, flexor digitorum longus, peroneus longus, and small extensor digitorum longus tendons. Muramatsu et al. documented an atypical case of GCTTS originating from the extensor hallucis longus tendon, affecting both feet equally.[32] The dorsal aspect of the foot was more commonly involved than the plantar aspect. Clinically, GCTTS usually presented as a slow-growing, painless, and firm mass with variable mobility.
| Tumors | Age | Gender | Clinical features | Histopathological features | Cellular patterns | Characteristics | |
|---|---|---|---|---|---|---|---|
| Nodular pattern | Molecular features | ||||||
| TSGCT | Young and middle aged-adults | Female predominance | LTSGCT predominantly occurs in the fingers and DTSGCT often occurs in the knee | LTSGCT: Well-circumscribed lesion DTSGCT: Poorly circumscribed lesion |
Small histiocyte-such as cells, larger epithelioid cells, and osteoclast-like giant cells | Fomay macrophages and desmin-positive cells | CSF1fusions (usually with COL6A3). |
| GCTST | Fifth decade | Equal male and female incidence. | Locally aggressive; Seen in superficial soft tissue of the extremities | Multi-nodular | Mononuclear cells and osteoclast-like giant cells. | Metaplastic bone formation | No H3-3Agene mutations |
| KPGCT | 2nd decade | Female predominance | Seen in superficial soft tissue of the extremities | Uninodular | Keratin-positive mononuclear cells and evenly distributed osteoclast-like giant cells | Peripheral inflammatory infiltrate | HMGA2-NCOR2fusions |
| UPS | Older adults | No distinct gender predominance | Seen in deep soft tissue of the extremities. Local recurrence, metastasis (common) |
Limited data | Atypical, pleomorphic cells with abundant mitotic figures Osteoclast-like multinucleated giant cells (10–15%) |
Infiltrative | No recurrent aberrations |
GCTST: Giant cell tumor of soft tissue, TSGCT: Tenosynovial giant cell tumor, KPGCT: Keratin-positive giant cell-rich tumor, UPS: Undifferentiated pleomorphic sarcoma, LTSGCT: Localized tenosynovial giant cell tumor, DTSGCT: Diffuse-type tenosynovial giant cell tumor, H3-3A: H3.3 histone A, CSF1: Colony-stimulating factor 1, COL6A3: Collagen type VI alpha 3 chain, HMGA2: High mobility group AT-hook 2, NCOR2: Nuclear receptor corepressor 2
CONSERVATIVE MANAGEMENT
In Stevenson et al.’s series,[11] four patients with diffuse-type pigmented villonodular synovitis of the foot and ankle were managed nonoperatively. These patients remained asymptomatic and under clinical and radiological surveillance, indicating that non-operative management can be a successful approach in selected cases where symptoms are minimal or absent. This highlights the importance of considering conservative approaches alongside surgical intervention, especially in asymptomatic patients.
Surgical management
The standard treatment for GCTTS in the foot is complete local excision [Figure 10]. Radiographs are crucial as they can reveal abnormal features such as cortical erosion of the bone and intraosseous involvement. MRI is essential for assessing the tumor’s size, extent, and relationship to surrounding structures. MRI typically shows a well-defined mass with low-signal intensity on both T1- and T2-weighted images, which helps differentiate GCTTS from other soft-tissue tumors (nerve sheath tumors, hemangiomas, and neurofibromas).
During surgery, meticulous dissection is required to protect nearby nerves and blood vessels. The use of magnification tools can aid in distinguishing between healthy tissue and tumor, especially in cases where the tumor lacks a well-defined capsule. Incomplete excision is the most significant risk factor for recurrence, as residual tumor cells can lead to regrowth. Therefore, ensuring clear margins is crucial, even if it necessitates a more extensive surgical approach.
Arthroscopy versus open surgery
The optimal treatment for GCTTS in the foot and ankle remains controversial, with choices influenced by tumor type, surgeon expertise, and available resources. While arthroscopic resection is gaining popularity for localized GCTTS in the knee, open surgery is often necessary for diffuse types and those with extra-articular involvement.[31-38]
| Recommendations | Level of evidence |
|---|---|
| Imaging | |
| Radiographs | B |
| MRI | B |
| Ultrasound | B |
| 18-FDG PET scans | C |
| Role of biopsy in diagnosis | I |
| Complete excision for benign tumors | A |
| Wide excision (aggressive) | C |
| Adjuvant treatment (radiotherapy) for recurrence in adults | C |
| Adjuvant treatment (radiotherapy) for recurrence in pediatric population | I |
According to Wright, grade A indicates good evidence (Level I studies with consistent findings) for or against recommending intervention; grade B, fair evidence (Level II or III studies with consistent findings) for or against recommending intervention; grade C, poor-quality evidence (Level IV or V studies with consistent findings) for or against recommending intervention; and grade I, insufficient or conflicting evidence not allowing a recommendation for or against intervention. MRI: Magnetic resonance imaging, 18-FDG PET: 18-fluoro-deoxyglucose (FDG) positron emission tomography
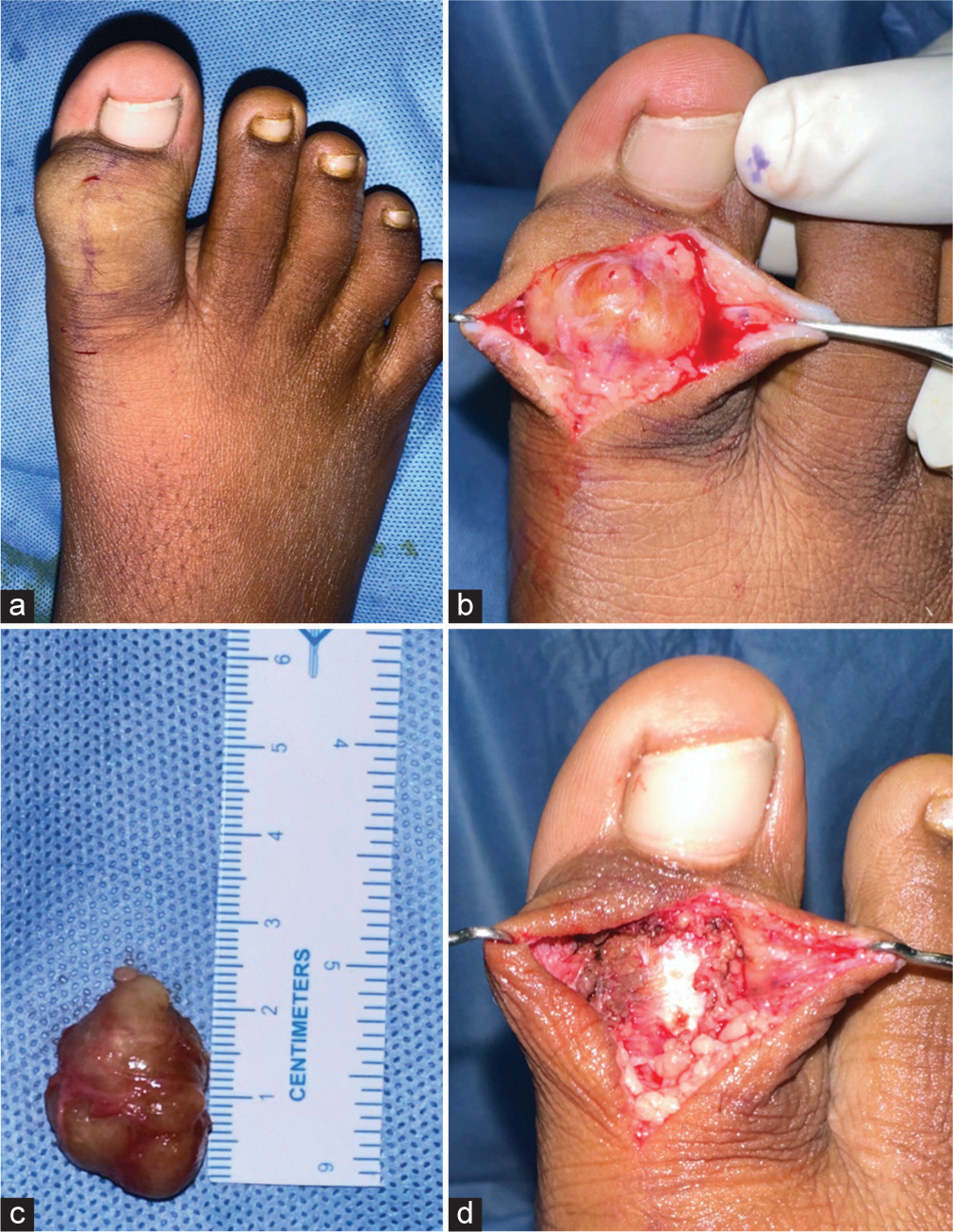
- (a) The giant cell tumor of the tendon sheath was identified in an 11-year-old boy’s right great toe. (b) Macroscopically, the lesion appeared as a well-circumscribed, orange-yellow nodule with a firm consistency. (c) The lesion measured approximately 2.5 cm in size length. (d) Complete tumor excision is feasible and can yield good results.
In the foot and ankle, arthroscopic resection is less common due to technical challenges and limited effectiveness, particularly for diffuse GCTTS. Recent studies suggest higher recurrence rates with arthroscopic treatment compared to open resection, particularly for diffuse cases. Therefore, open surgery is generally recommended, especially for diffuse GCTTS with extra-articular involvement.
However, the high recurrence rates and complications associated with both surgical approaches highlight the need for further research into alternative treatments, such as systemic therapy, which may be beneficial for refractory or recurrent cases.
Complications
Complications are more frequent in diffuse GCTTS (24%) than localized GCTTS (12%). Foot and ankle involvement may have a higher risk due to poorer blood circulation, which can be exacerbated by factors such as nicotine abuse, atherosclerosis, and diabetes, impacting healing potential.[31-38] In addition, hygienic challenges in foot surgery can contribute to complications.
Time to recurrence
In cases of diffuse-type GCTTS, 79.3% of documented recurrences occurred with an average recurrence time of 33.8 months after initial treatment, ranging from 3 to 144 months.[27,31-38] Most of these recurrences were observed within the first two years, with a notable second peak around four years (18%). In addition, some late recurrences occurred beyond 84 months, with the latest at 144 months. In contrast, the localized type of GCTTS has a significantly lower recurrence rate of 7%. Among these, 84.6% of recurrences were documented, with 72.7% occurring within the first three years. The mean time to recurrence for localized GCTTS was 55.6 months, ranging from 1 to 264 months. Notably, some late recurrences were reported at 9, 12, and even 22 years post-treatment.[27,31-38] This data highlights the importance of long-term monitoring for patients with GCTTS, especially those with the diffuse type.
Post-operative management and recurrence
Post-operative care involves monitoring for signs of recurrence through regular follow-up and imaging studies. The local recurrence rate of GCTTS varies widely, from 9% to 45%. Zhang et al.[27] observed a 20% recurrence rate, with recurrences occurring within an average of 5.3 months post-surgery. Factors predictive of recurrence include incomplete excision, pressure erosions on radiographs, interphalangeal joint location, and the presence of degenerative joint disease. However, some studies, such as Lowyck and De Smet,[29] found no significant correlation between these factors and recurrence rates.
Adjuvant therapies, such as pre-operative radiotherapy, have been explored to reduce recurrence rates, but their efficacy remains controversial. They are generally not recommended due to potential complications and lack of conclusive benefits. GCTTS should be considered in patients presenting with foot or ankle swelling. Accurate pre-operative diagnosis and complete local excision are essential to prevent recurrence. Post-operative adjuvant radiotherapy, although explored by some investigators, is not routinely recommended due to mixed efficacy results and potential side effects.
FOLLOW-UP REGIME
Diffuse type
Initial clinical and radiological (MRI) follow-up three months post-intervention
Twice-yearly clinical and radiological follow-ups for two years
Annual clinical and radiological examinations until the 5th year
Individualized follow-up beyond five years based on clinical course
Follow-up outside this schedule for rapidly increasing symptoms.
Localized type
Early clinical and radiological follow-up 3–6 months post-surgery
Annual clinical and radiological (if symptomatic) follow-up for three years
Individualized follow-up based on clinical symptoms thereafter.
CONCLUSION
GCTTS is a benign yet complex entity characterized by nonspecific symptoms and a typically slow progression. Accurate diagnosis necessitates MRI and often histological confirmation. Treatment urgency is not a hallmark of the tumor, allowing for thoughtful consideration of individualized approaches based on symptomatology, progression, location, and patient factors.
When surgical intervention is warranted, the aim should be complete resection, especially in primary cases. Targeted therapies such as isotopic synoviorthesis and external RT, while less commonly employed, may hold promise in specific scenarios and should be evaluated on a case-by-case basis, particularly for recurrent diseases.
GCTTS is a prevalent benign tumor of the hand and foot, and accurate diagnosis and appropriate treatment are essential to avoid potential complications such as amputation. Meticulous surgical excision under magnification in a bloodless field remains the gold standard of care. While targeted synovectomy without adjuvant radiotherapy can yield excellent outcomes in some cases, asymptomatic patients may be successfully managed nonoperatively. Further research is warranted to refine treatment algorithms and optimize outcomes for patients with this diverse group of tumors.
AUTHORS’ CONTRIBUTIONS
JTJJ and DK - Conceptualization, methodology/study design, software, validation, formal analysis, investigation, resources, data curation, writing original draft, writing review and editing, visualization, supervision, project administration, funding acquisition. DK - Drawing. All authors have critically reviewed and approved the final draft and are responsible for the manuscript’s content and similarity index.
ETHICAL APPROVAL
This study was approved by the Institutional Review Board (IRB) under protocol number OHRC 45/2024, dated 15 June 2024.
DECLARATION OF PATIENT CONSENT
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published, and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY FOR MANUSCRIPT PREPARATION
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
CONFLICTS OF INTEREST
There are no conflicting relationships or activities.
FINANCIAL SUPPORT AND SPONSORSHIP
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Diffuse and localized tenosynovial giant cell tumor and pigmented villonodular synovitis: A clinicopathologic and flow cytometric DNA analysis. Hum Pathol. 1992;23:729-35.
- [CrossRef] [PubMed] [Google Scholar]
- Localized and diffuse forms of tenosynovial giant cell tumor (formerly giant cell tumor of the tendon sheath and pigmented villonodular synovitis) Orthop Traumatol Surg Res. 2017;103:S91-7.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnosis and treatment of pigmented villonodular synovitis. J Bone Joint Surg Br. 1968;50:290-305.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumours of tendon sheath: Classification and recurrence rate. J Hand Surg Br. 2001;26:72-5.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant giant cell tumor of the tendon sheaths and joints (malignant pigmented villonodular synovitis) Am J Surg Pathol. 1997;21:153-63.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of surgery for giant cell tumors of the tendon sheath within the hand. Pol Przegl Chir. 2020;92:16-21.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of soft tissue: An updated review. J Clin Med. 2024;13:2870.
- [CrossRef] [PubMed] [Google Scholar]
- Metastasizing tenosynovial giant cell tumour, diffuse type/pigmented villonodular synovitis. Clin Sarcoma Res. 2015;5:15.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented villonodular synovitis of joints. J Surg Oncol. 2011;103:386-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented villonodular synovitis of the foot and ankle: A report of eight cases. Foot Ankle Int. 1999;20:587-90.
- [CrossRef] [PubMed] [Google Scholar]
- Diffuse pigmented villonodular synovitis (diffuse-type giant cell tumour) of the foot and ankle. Bone Joint J. 2013;95B:384-90.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumour of tendon sheath of the digits. A systematic review. Hand (N Y). 2011;6:244-9.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence of giant cell tumors in the hand: A prospective study. J Hand Surg Am. 2010;35:451-6.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of tendon sheath: Study of 64 cases and review of literature. G Chir. 2013;34:149-52.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of tendon sheath in the hand: Analysis of risk factors for recurrence in 50 cases. BMC Musculoskelet Disord. 2019;20:457.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of surgical synovectomy and radiotherapy on the rate of recurrence of pigmented villonodular synovitis of the knee: An individual patient meta-analysis. Bone Joint J. 2015;97B:550-7.
- [CrossRef] [PubMed] [Google Scholar]
- Giant-cell tumour of the tendon sheath. Is radiotherapy indicated to prevent recurrence after surgery? J Bone Joint Surg Br 2000. ;. ;82:571-3.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcome of the treatment of high-risk tenosynovial giant cell tumor/pigmented villonodular synovitis with radiotherapy and surgery. Cancer. 2012;118:4901-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented villonodular synovitis: A retrospective single-center study of 122 cases and review of the literature. Semin Arthritis Rheum. 2011;40:539-46.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented villonodular synovitis in children: Review of six cases. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:64-72.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome following radiation treatment for high-risk pigmented villonodular synovitis. Int J Radiat Oncol Biol Phys. 1995;32:777-86.
- [CrossRef] [PubMed] [Google Scholar]
- A large, multiply recurrent tenosynovial giant cell tumor of the hand treated with resection and radiation therapy. Am J Orthop (Belle Mead NJ). 2004;33:137-40.
- [Google Scholar]
- Recurrent Tenosynovial Giant Cell Tumor (TGCT) in thumb treated by excision and adjuvant 3DCRT radiation. Indian J Orthop. 2022;56:1469-73.
- [CrossRef] [PubMed] [Google Scholar]
- Diffuse tenosynovial giant cell tumor of the wrist with joint destruction and invasion. J Arthrosc Joint Surg. 2024;11:41-5.
- [CrossRef] [Google Scholar]
- Benign fibrous histiocytoma of tendon sheath in the index finger of a 70-year-old patient--A case report. Indian J Surg Oncol 2024 doi: 10.1007/s13193-024-01971-5
- [CrossRef] [PubMed] [Google Scholar]
- CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: A dose-escalation and dose-expansion phase 1 study. Lancet Oncol. 2015;16:949-56.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of the tendon sheath in the foot and ankle: Case series and review of the literature. J Foot Ankle Surg. 2013;52:24-7.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrent giant cell tumors of the tendon sheath. J Hand Surg Am. 1999;24:1298-302.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence rate of giant cell tumors of the tendon sheath. Eur J Plast Surg. 2006;28:385-8.
- [CrossRef] [Google Scholar]
- Giant cell tumour of tendon sheath: Experience with 52 cases. Singapore Med J. 2008;49:879-82.
- [Google Scholar]
- Giant cell tumor of tendon sheath arising in the toe. Fetal Pediatr Pathol. 2004;23:171-9.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical tenosynovial giant cell tumor of the extensor hallucis longus tendon. J Am Podiatr Med Assoc. 2006;96:359-61.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration cytology of giant cell tumor of soft tissue (soft tissue giant cell tumor of low malignant potential) Ann Diagn Pathol. 2003;7:365-9.
- [CrossRef] [PubMed] [Google Scholar]
- Fibrous xanthoma of synovium (giant-cell tumor of tendon sheath, pigmented nodular synovitis). A study of one hundred and eighteen cases. J Bone Joint Surg Am. 1969;51:76-86.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumor of tendon sheath in the hand and wrist. J Hand Microsurg. 2020;15:236-7.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment, recurrence rates and follow-up of Tenosynovial Giant Cell Tumor (TGCT) of the foot and ankle-A systematic review and meta-analysis. PLoS One. 2021;16:e0260795.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of tenosynovial giant cell tumor of the foot and ankle. Foot Ankle Int. 2023;44:1013-20.
- [CrossRef] [PubMed] [Google Scholar]
- Best clinical management of Tenosynovial Giant Cell Tumour (TGCT): A consensus paper from the community of experts. Cancer Treat Rev. 2023;112:102491.
- [CrossRef] [PubMed] [Google Scholar]
- Revised grades of recommendation for summaries or reviews of orthopaedic surgical studies. J Bone Joint Surg Am. 2006;88:1161-2.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics and treatment outcomes in six cases of malignant tenosynovial giant cell tumor: Initial experience of molecularly targeted therapy. BMC Cancer. 2018;18:1296.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant diffuse-type tenosynovial giant cell tumors: A series of 7 cases comparing with 24 benign lesions with review of the literature. Am J Surg Pathol. 2008;32:587-99.
- [CrossRef] [PubMed] [Google Scholar]
- Measurement of invasive potential provides an accurate prognostic marker for giant cell tumour of tendon sheath. J Hand Surg Br. 1998;23:728-31.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented villonodular synovitis of the hand in the Asian population. Hand Surg. 1999;4:81-5.
- [CrossRef] [PubMed] [Google Scholar]
- Pigmented villonodular synovitis (giant-cell tumor of the tendon sheath and synovial membrane). A review of eighty-one cases. J Bone Joint Surg Am. 1984;66:76-94.
- [CrossRef] [PubMed] [Google Scholar]
- Giant cell tumour of the tendon sheath involving the hand or the wrist: An analysis of 142 patients. Acta Orthop Traumatol Turc. 2004;38:120-4.
- [Google Scholar]






