Translate this page into:
Bilateral hip ochronosis, an unsuspected diagnosis in polyarticular pain: A case report
*Corresponding author: Camilo A. Delgadillo, Department of Orthopedics and Traumatology, University Hospital Fundacion Santa Fe de Bogota, Bogota, Colombia. camilo.delgadillo@urosario.edu.co
-
Received: ,
Accepted: ,
How to cite this article: Gomez Mier L, Arroyave Rivera SA, Delgadillo C, Melo-Uribe M. Bilateral hip ochronosis, an unsuspected diagnosis in polyarticular pain: A case report. J Musculoskelet Surg Res. 2025;9:128-33. doi: 10.25259/JMSR_232_2024
Abstract
Alkaptonuria is a rare metabolic disorder characterized by the accumulation of homogentisic acid, leading to the deposition of blackish pigment in cartilage and soft tissue, known as ochronosis. This can mimic multiple joint pathologies and is a diagnosis of exclusion. We describe the case of a 52-year-old woman who had a total hip replacement after experiencing left hip pain for which oral medications were unable to provide relief. Unexpectedly, during the procedure, a blackish tissue was noticed. This led to a histological examination and the confirmation of the ochronosis diagnosis. The patient’s medical history revealed a number of malignancies, but no previous metabolic illness diagnoses. Subsequent follow-up revealed rapid-onset pain in other joints, leading to a similar diagnosis of polyarticular ochronosis in the right hip, knees, spine, and shoulders. This highlights how difficult it is to treat this condition. This case underscores the importance of considering ochronosis as a differential diagnosis in patients with chronic polyarticular pain. Current treatment consists of initial non-surgical measures, including physical therapy and analgesics. If there is no improvement, treatment would be joint arthroplasty. Awareness among orthopedic surgeons and histopathological examination of tissues is crucial for the accurate diagnosis and management of ochronosis.
Keywords
Alkaptonuria
Case report
Homogentisic acid
Ochronosis
Polyarticular
INTRODUCTION
Alkaptonuria (AKU, MIM #203500) is an inherited metabolic disorder with a very low frequency; a prevalence of 250,000–1,000,000 is suspected around the world.[1] The AKU Society, which is based in the United Kingdom, has only listed 1233 patients.[2] It was first observed by Scribonius in 1584 in a boy with black urine.[3] AKU is characterized by the accumulation of homogentisic acid (HGA),[3] an intermediate metabolic product of the tyrosine and phenylalanine metabolism pathways, due to a Mendelian autosomal recessive deficiency of the enzyme homogenistate 1,2-dioxygenase (HGD), mapped on long arm chromosome 3q21-q23, within 14 exons,[1,4] expressed predominantly in the liver and kidney, and other organs, such as the gallbladder, gastrointestinal tract, prostate, thyroid, and cartilage.[2,4] To date, 212 HGD gene variants have been reported;[2] the missense variants represent around 65% of all AKU mutations, followed by splicing (13.4%) and frameshift (11.3%) mutations,[2] each of which has a different impact on the disease expression.[5] Furthermore, severity depends on whether there is a homozygous or heterozygous mutation of the HGD.[6] The typical manifestation is known as the triad of AKU (excess HGA oxidizes and turns dark when standing), ochronosis (HGA oxidation products deposit in connective tissue, causing it to discolor), and ochronotic arthropathy (accumulation of HGA plaques causes brittleness and eventually arthritis).[7] The abnormal accumulation of pigments in the cellular matrix can elicit changes in the consistency of the cartilage, thickening, and stiffness of the tendon and ligaments, along with posterior articular instability, which can lead to articular degeneration.[2,8] This results in oxidative stress that causes tissue damage and loss of ligament tensile strength with increased joint mobility due to instability.[2,8] The objective of this case report of an inadvertent diagnosis of ochronosis is to highlight that, despite its low prevalence, it is important to keep this disease in mind as a possible cause of secondary osteoarthritis.
CASE REPORT
We present the case of a 52-year-old woman with pain in her left hip without associated trauma. She required crutches for ambulation. Medical history showed left breast cancer, nasal bridge squamous cell carcinoma, and gastrointestinal stromal tumor; all were treated with resection and chemotherapy; no radiotherapy was used. Physical examination revealed a decrease in the full range of motion of the left hip. Initial radiographs showed erosion of the hip surface, and magnetic resonance imaging (MRI) ruled out secondary bone malignancy [Figure 1]. A total hip arthroplasty was indicated. During the intraoperative procedure, a blackish discoloration of the articular cartilage was observed [Figure 2]. The affected cartilage appeared more brittle and discolored than expected, raising concerns about an underlying metabolic disorder. The histopathological examination revealed the presence of intense black pigmentations within the extracellular matrix, confirming the diagnosis of ochronosis [Figure 3]. Posteriorly darkish sclerae were visualized (Osler sign), with darkish pigment in bilateral pinna [Figure 4]; dental pigmentation was also observed. After 1 year, the patient returns for a rapid onset of pain in her right hip, knees, shoulders, and lumbar pain. Radiographs showed fragmentation and subluxation of the right hip [Figure 5], as well as severely decreased joint space in the knees and shoulders. Infectious or malignant causes were ruled out. Bone scintigraphy showed increased uptake in the bilateral shoulder, right hip, bilateral knees, pubis, lumbar, and cervical spine. A total hip arthroplasty was performed with similar macroscopic and microscopic findings. Initial physical therapy and analgesics were used for knee and shoulder osteoarthritis. After one year of follow-up, the bilateral cementless hip prostheses were found without signs of loosening; the right shoulder pain progressed in intensity, requiring treatment with analgesic infiltrations due to the desire for no additional arthroplasties. The final AKU Severity Score Index (AKUSSI) after 3 years of follow-up of the patient was 104 points.
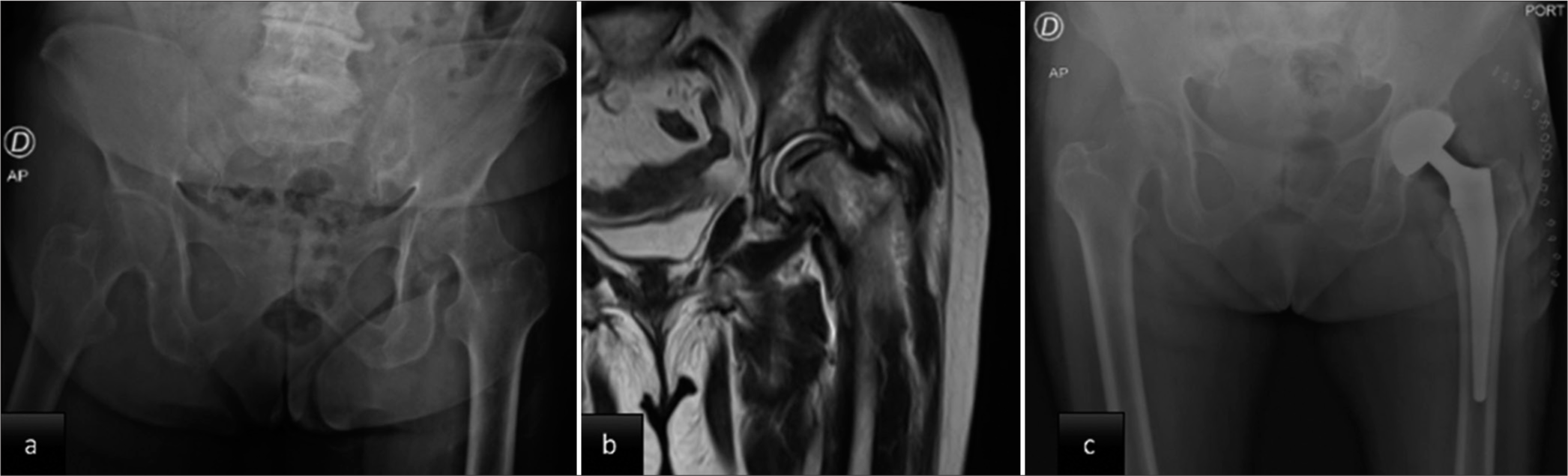
- (a) Initial hip radiograph shows severe osteoarthritic changes with decreased joint space and a change in the shape of the femoral head. (b) Magnetic resonance imaging of the left hip: Decreased articular cartilage without evidence of bone tumor lesions. (c) Post-operative hip radiograph: cementless total hip prosthesis.
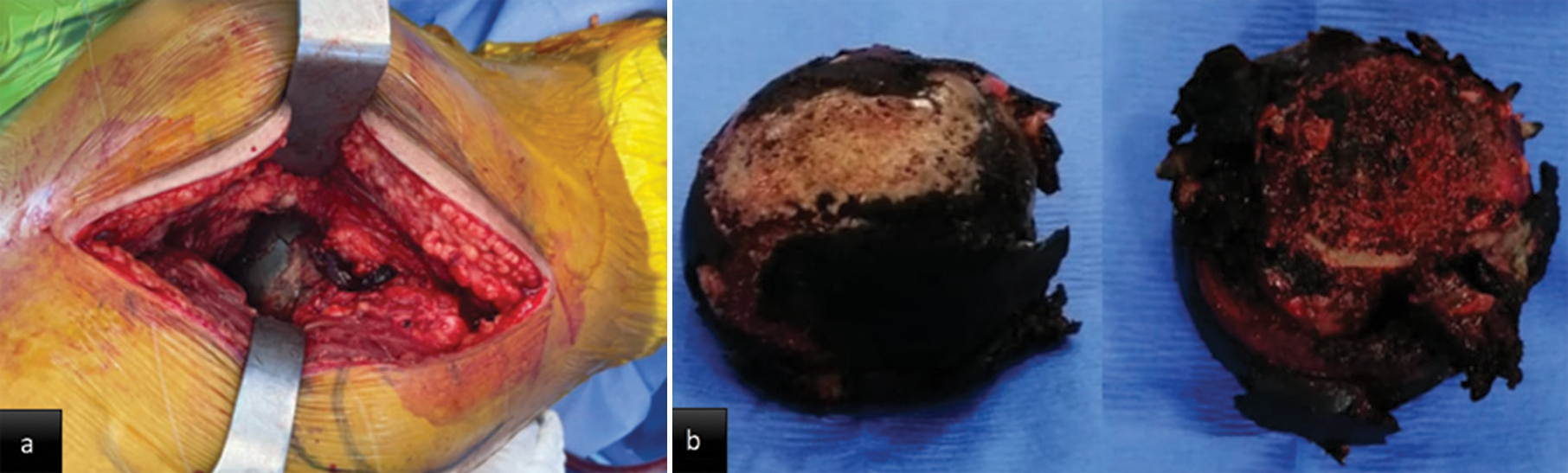
- Intraoperative images: (a) Dark pigment in acetabular cartilage (b) dark pigment in the femoral head.
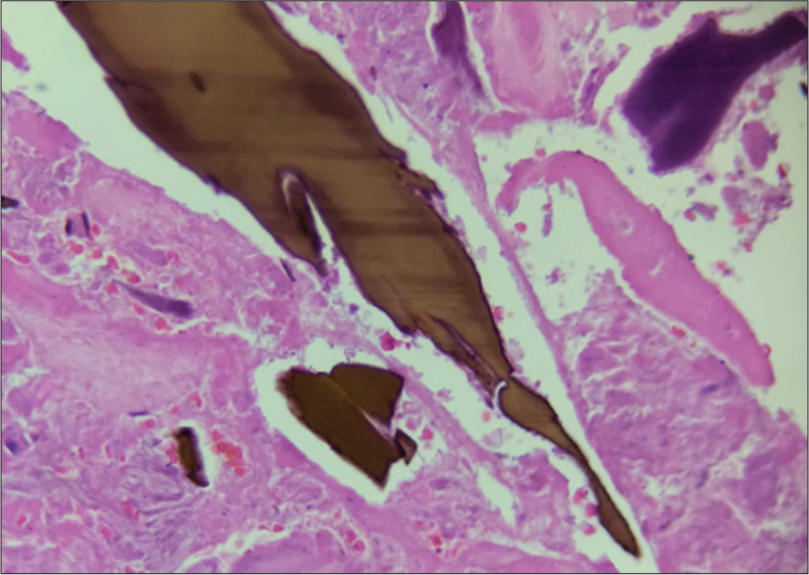
- Microscopic findings: Ochronotic bodies deposit in the connective tissue yellow-brown in sickled shape.
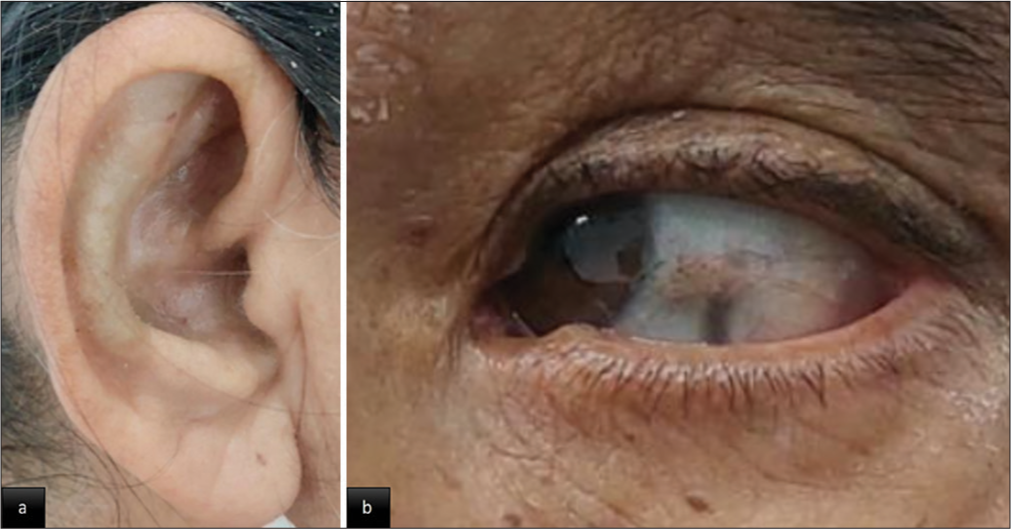
- Clinical images with blue-black pigmentation in (a) antitragus of the ear. (b) Sclera of the left eye (Osler sign).
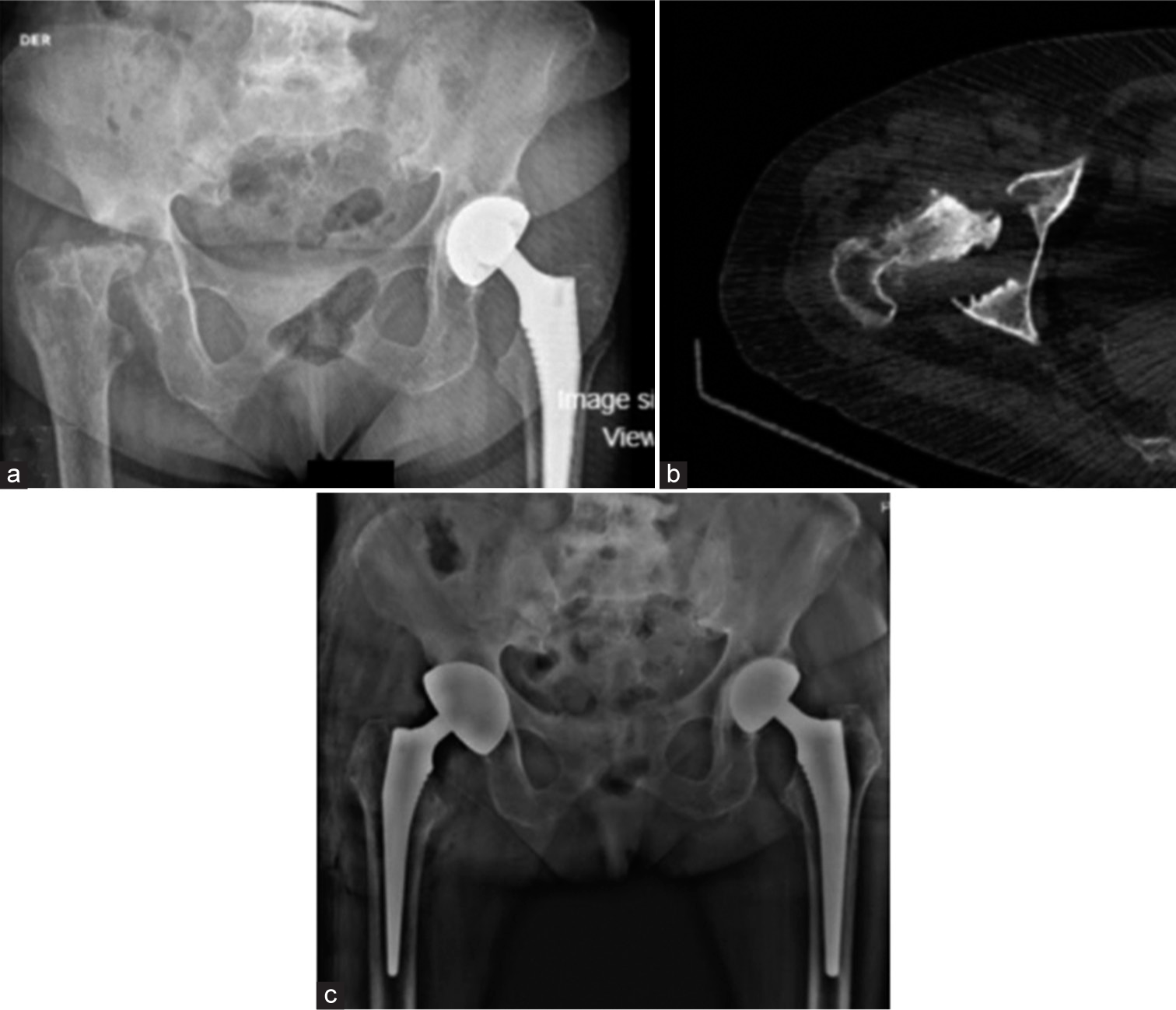
- (a) Bilateral hip radiograph, 1 year postoperatively: Left hip prosthesis without changes, bone resorption changes in the proximal femur head and right supra-acetabular. (b) Computed axial tomography confirming acetabular and femoral head erosion. (c) Post-operative hip radiograph: Uncemented mega acetabular cup and femoral stem.
DISCUSSION
Ochronosis is a metabolic disorder that affects the musculoskeletal system, leading to pigmentation and degeneration of cartilaginous tissues. The cascade of pigment deposition begins in the area of calcified cartilage, which causes a focal increase in rigidity and changes the distribution of the articular load.[2] The ochronotic pigment creates a redox environment, increases collagen degradation,[9] and is bound to type II collagen fibers and proteoglycans with final deposition of type III collagen by chondrocytes.[2,10] Subsequently, extension to the hyaline cartilage, due to its rigidity, is not capable of supporting loads, with posterior resorption of the subchondral plate, decreasing the ability to produce proteoglycans and lubricant production of the superficial layer.[2,6,10] In this case, the clinic presentation of polyarticular joint pain and degenerative changes on radiographs initially pointed toward a diagnosis of primary osteoarthritis after ruling out secondary bone malignancies. However, the incidental intraoperative finding of blackish cartilage led to suspicion and post-operative histopathological confirmation.
The age at which symptoms appear depends on the origin of the mutation, the amount of residual functional protein, and kidney function. Dark urine may be the initial symptom and even the only reason for parents to consult.[10,11] Our patient, despite her oncological history, which was under strict follow-up, had not reported symptoms of joint pain or changes in urine color. Possibly because for it to occur, the urine must be alkaline, or sodium hydroxide or ferric chloride must be added for it to change. In a state of normal HGD function, the liver can convert around 1.5 kg of HGA daily; a loss of 99% of HGD enzyme activity is required before patients become symptomatic.[11,12] The literature reports that symptoms appear around the third decade of life,[10] with skin hyperpigmentation, especially in the sclerae and subcutaneous cartilage of the ear [Figure 4].[10] Must be suspected in patients with low back pain and polyarticular pain before 40 years.[13] The order of joint involvement is first the knees, then the hips, and the shoulders. The erosive deterioration of the joint could happen months after the initial onset of the pain.[14] Extraosseous symptoms are cardiac involvement and decreased kidney function, not failure,[15] which can accelerate the deposits.[10,11] Medical reports do not show any extra-osseous symptoms in our case.
Diagnosis methods might vary greatly in scope, such as identifying ochronotic bodies in the extracellular matrix [Figure 3]. We did not perform the urine analysis. Other specific testing includes measuring the excretory level of HGA for 24 h,[16] as well as chromatographic, enzymatic, or spectrophotometric determinations of HGA;[16] molecular studies for the detection of three mutations of the HGD gene,[17] not always available. The differential diagnosis includes osteoarthritis, ankylosing spondylitis, rheumatoid arthritis, and calcium pyrophosphate arthropathy.[11] Pseudoochronosis has been described as a result of argyria or after long-term use of L-dopa, methyldopa, antimalarial agents, or products containing hydroxychloroquine, phenol, resorcinol, and mercury or picric acid.[11] Our patient did not receive any type of these medications.
The musculoskeletal symptoms analysis begins with radiographic studies, which show degenerative joint changes with decreased joint space, subchondral sclerosis, and joint collapse with osteopenia [Figure 1] with calcification of soft tissues (such as menisci, ligaments, and tendons).[11,12] In the spine, radiologic findings are a narrowing of the intervertebral spaces with vacuum phenomenon, and wafer-like disc calcification with posterior bridges between adjacent vertebrae.[11,18] An MRI was performed on our patient based on the oncological history to rule out the possibility of a secondary bone tumor. The results indicated involvement of the subchondral bone and articular cartilage, along with surrounding edema.
The historical treatment of the disease is primarily supportive, focused on symptom relief with non-steroidal anti-inflammatories, glucosamine, chondroitin sulfate and functional improvement with physical therapy. Unfortunately, there is no prophylactic treatment for alkaptonuric patients. Invasive procedures include intra-articular injection of hyaluronic acid and steroids; arthroscopic debridement of the affected joint can relieve symptoms for at least 18 months;[12] in cases of severe symptoms, the arthroplasty may be necessary to alleviate pain and restore function. During arthroplasty, special caution must be taken regarding the risk of fractures when introducing the components due to the secondary osteopenia of the disease. Likewise, evidence shows no difference in survival outcomes between cemented and non-cemented components, with follow-up for more than 10 years.[19-21] There are no reports of increased risk of periprosthetic infection. Approximately 50% of patients aged 55 years require at least one joint replacement surgery.[13] In a case series of 58 patients, 13% required three or more arthroplasties.[12] At older ages, a greater number of joint replacements are observed; in addition, men have been found to have symptoms earlier and of greater intensity, with more severe radiological findings.[10]
As mentioned previously, there is no way to prevent the disease, but there are some ways to limit its progression. The literature suggests that diet adjustments, particularly anti-inflammatory diets rich in Vitamin C, can reduce the conversion of HGA to its pathogenic polymeric intermediate, is more useful in children than adolescents and adults.[10,12] Dietary limitations on foods containing phenylalanine and tyrosine have been proposed to be useful in minimizing symptoms of ochronotic arthritis because they diminish HGA synthesis.[22,23] Phornphutkul et al.[10] showed that in cases who take vitamin C (dose reported, 0.25–4.0 g/day), the mean urinary HGA level was 3.17 ± 1.11 mmol/millimole of creatinine (range, 1.8–6.1), but it was similar to the level untreated patients (3.00 ± 1.01 mmol/millimole of creatinine [range, 1.0–5.5]); and patients adhered to a low-protein diet; the mean urinary HGA level in these patients was 3.93 ± 1.63 mmol/millimole of creatinine (range, 1.8–6.6). Furthermore, it has been proposed the use of other antioxidants, such as Vitamin E and N-acetyl cysteine, which may theoretically scavenge the damaging oxygen free radicals, and antiresorptive therapy has been proposed as an early prophylactic intervention of fractures secondary to osteopenia.[12]
In light of new technologies, with advances in the understanding of pathophysiology and multiple mutational variables, new therapies aim to reduce HGA production in the liver. These include liver transplantation, or administration of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione or nitisinone.[2,12,24] This is an herbicide that inhibits 4-hydroxyphenylpyruvate dioxygenase, responsible for the conversion of 4-hydroxyphenylpyruvate to HGA.[24] A dose of 2 mg daily decreased urine HGA by >80% and slowed the progression of AKU.[2,24] The principal adverse effect is elevated levels in the plasma of tyrosine, which generates toxic effects on the skin, nervous system, and keratopathy. It is important to restrict dietary consumption of tyrosine and phenylalanine during the use of this nitisinone.[5,22,24] Studies are being carried out on non-sense variants; in theory, attacking HGD with pharmacological chaperones (small molecules that help with structural stability) has predicted the rescue of enzymatic activity.[12]
The follow-up of these patients should be carried out closely, including the use of AKUSSI; this score of 264 points, which encompasses the totality of clinical and radiologic findings, is sensitive to all morbid features of AKU. It has been shown that the higher the score, the greater the severity of the disease. It is also useful to assess the clinical response to treatment, as in the case of nitisinone.[2] Unfortunately, in our case, treatment with arthroplasty and pharmacological measures do not stop the progression of the disease, which is why the global initiation of existing medications is needed for these patients, hence the importance of registration in patient societies, such as the AKU Society, to advise patients and reduce the under-reporting of this condition.
CONCLUSION
This case of incidental ochronosis discovered during total hip arthroplasty in a 52-year-old woman presenting with chronic pain in both hips highlights the importance of considering ochronosis as a differential diagnosis in patients with chronic joint pain. Orthopedic surgeons should be aware of the possibility of ochronosis during surgical interventions, and histopathological examination of suspicious tissue can aid in the confirmation of the diagnosis.
RECOMMENDATIONS
In cases of low back and polyarticular pain in patients around the third decade of life, in which the diagnosis of ochronosis is confirmed, it is important to continue cardiac and renal follow-up. Keeping in mind that the cause of the symptoms is joint degeneration; so far, joint arthroplasty and changes in diet are the treatment methods in most cases. Patients need to register with the AKU Societies.
AUTHORS’ CONTRIBUTIONS
CAD and SAAR designed the report, reviewed the literature, and wrote the initial and final drafts. LCGM suggested the idea of the study and was the primary surgeon. CAD and SAAR organized and prepared the figures. MMU performed the histopathological examination of the specimens. All authors have critically reviewed and approved the final draft and are responsible for the manuscript’s content and similarity index.
ETHICAL APPROVAL
The Institutional Review Board approval is not required.
DECLARATION OF PATIENT’S CONSENT
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has consented for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published, and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY FOR MANUSCRIPT PREPARATION
During the preparation of this work, the authors used QuillBot in order to edit the manuscript. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
CONFLICTS OF INTEREST
There are no conflicting relationships or activities.
FINANCIAL SUPPORT AND SPONSORSHIP
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Ochronotic arthritis and ochronotic Achilles tendon rupture in alkaptonuria: A 6 years follow-up case report in China. Medicine (Baltimore). 2019;98:e16837.
- [CrossRef] [Google Scholar]
- Ochronotic arthritis of bilateral knees: A case report. Int J Clin Exp Med. 2015;8:8185-9.
- [Google Scholar]
- Homogentisate 1,2-dioxygenase (HGD) gene variants in young Egyptian patients with alkaptonuria. Sci Rep. 2023;13:14374.
- [CrossRef] [Google Scholar]
- Analysis of the phenotype differences in siblings with alkaptonuria. Metabolites. 2022;12:990.
- [CrossRef] [Google Scholar]
- Alkaptonuric ochronosis with aortic valve and joint replacements and femoral fracture: A case report and literature review. Clin Med Res. 2004;2:209-15.
- [CrossRef] [Google Scholar]
- Alkaptonuria In: The metabolic basis of inherited disease. New York: McGraw Hill; 1978. p. :268-80.
- [Google Scholar]
- Homogentisic acid induces morphological and mechanical aberration of ochronotic cartilage in alkaptonuria. J Cell Physiol. 2019;234:6696-708.
- [CrossRef] [Google Scholar]
- Development of an effective therapy for alkaptonuria-lessons for osteoarthritis. Rheumatol Immunol Res. 2021;2:79-85.
- [CrossRef] [Google Scholar]
- Orthopedic manifestations of ochronosis: Pathophysiology, presentation, diagnosis, and management. Am J Med. 2016;129:6.e1-6.
- [CrossRef] [Google Scholar]
- Ochronosis: A report of three cases and review of the literature. Indian J Pathol Microbiol. 2011;54:626-8.
- [CrossRef] [Google Scholar]
- Devastating ochronotic arthropathy with successful bilateral hip and knee arthroplasties. J Clin Rheumatol. 2009;15:138-40.
- [CrossRef] [Google Scholar]
- Impact of chronic kidney disease on the natural history of alkaptonuria. Clin Kidney J. 2012;5:352-5.
- [CrossRef] [Google Scholar]
- Identification of potential inhibitors for the treatment of alkaptonuria using an integrated in silico computational strategy. Molecules. 2023;28:2623.
- [CrossRef] [Google Scholar]
- Quick diagnosis of alkaptonuria by homogentisic acid determination in urine paper spots. JIMD Rep. 2017;31:51-6.
- [CrossRef] [Google Scholar]
- A mimic of ankylosing spondylitis, ochronosis: Case report and review of the literature. Curr Allergy Asthma Rep. 2021;21:19.
- [CrossRef] [Google Scholar]
- Ochronotic arthropathy: Arthroscopic findings in the shoulder and the knee. Arthroscopy. 2003;19:E14-7.
- [CrossRef] [Google Scholar]
- Arthroplasty for ochronotic arthritis: No failure of 11 replacements in 3 patients followed 6-12 years. Acta Orthop Scand. 2004;75:355-8.
- [CrossRef] [Google Scholar]
- Long-term follow-up of bilateral hip and knee arthroplasty secondary to ochronotic arthropathy. Arthroplast Today. 2020;6:214-9.
- [CrossRef] [Google Scholar]
- Dietary restriction of tyrosine and phenylalanine lowers tyrosinemia associated with nitisinone therapy of alkaptonuria. J Inherit Metab Dis. 2020;43:259-68.
- [CrossRef] [Google Scholar]
- The nutritional status of people with alkaptonuria: An exploratory analysis suggests a protein/energy dilemma. JIMD Rep. 2020;53:45-60.
- [CrossRef] [Google Scholar]
- Efficacy and safety of once-daily nitisinone for patients with alkaptonuria (SONIA 2): An international, multicentre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2020;8:762-72.
- [CrossRef] [Google Scholar]







