Translate this page into:
Combined distal tibial nerve transfer and tibialis posterior tendon transfer for foot drop correction: A surgical technique and case illustration
*Corresponding author: J. Terrence Jose Jerome, FRCS., EDHS. Department of Orthopaedics, Hand and Reconstructive Microsurgery, Olympia Hospital and Research Centre, Trichy, Tamil Nadu, India. terrencejose@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jerome JTJ, Karunanithi D. Combined distal tibial nerve transfer and tibialis posterior tendon transfer for foot drop correction: A surgical technique and case illustration. J Musculoskelet Surg Res. 2025;9:145-52. doi: 10.25259/JMSR_264_2024
Abstract
Tendon transfers have traditionally been the mainstay for treating foot drop and improving gait and mobility. Recently, distal nerve transfers from the tibial to the deep peroneal nerve have been explored, but with limited success in achieving ankle dorsiflexion. We present a novel surgical technique combining both distal tibial nerve transfers (to both deep and superficial peroneal nerves) and tibialis posterior tendon transfer to the tibialis anterior (internal splint). This dual approach aims to achieve comprehensive foot drop correction through the reinnervation of foot dorsiflexors and improved ankle function. A case illustration highlights this combined technique’s surgical steps and potential benefits.
Keywords
Combined approach
Deep peroneal nerve
Foot drop
Nerve transfer
Superficial peroneal nerve
Surgical technique
Tendon transfer
Tibial nerve
Tibialis posterior
INTRODUCTION
Foot drop, resulting from sciatic or common peroneal nerve injuries, significantly impacts gait and quality of life.[1-4] Conventionally, treatment options have included nerve repair with grafts and tendon transfers.[3-5] While nerve grafts can be effective for shorter defects, longer grafts often yield less satisfactory outcomes.[3] While improving function and patient satisfaction, tendon transfers may not fully restore strength and balance and carry long-term risks of flatfoot, hindfoot valgus deformity, and arthritis.[4-6]
Nerve transfers have emerged as a promising alternative, leveraging the body’s capacity for reinnervation by connecting healthy nerve fibers to damaged ones near the target muscles.[7] Although well-established in upper extremity reconstruction, lower extremity nerve transfers are still evolving, with limited clinical evidence and variable outcomes.[8] This variability has highlighted the need to identify predictive factors for success, as some patients experience significant improvement in dorsiflexion strength while others see minimal benefit.
The choice of donor’s nerve for reinnervating foot drop muscles is also a subject of ongoing investigation. Various options have been explored, including branches from the tibial nerve and its motor branches, but their relative efficacy remains unclear.[6-8]
Given these complexities, this article proposes an integrated approach combining distal nerve transfers with tibialis posterior tendon transfer to the tibialis anterior. By targeting both reinnervation and mechanical support, this dual strategy aims to enhance functional outcomes, improve dorsiflexion strength, and ultimately achieve greater patient satisfaction.
INDICATIONS
The combined approach of distal tibial nerve transfers and tibialis posterior tendon transfer is indicated for patients with foot drop resulting from sciatic or common peroneal nerve injuries of <18 months. This technique is also applicable for complete or partial peroneal nerve palsy when proximal dissection and isolation of the motor component are feasible. Patients desiring a comprehensive foot drop correction, aiming to restore both ankle and foot dorsiflexion, can be considered. However, ensuring adequate donor nerve and tendon availability is crucial before proceeding with this approach.
CONTRAINDICATIONS
The tibial nerve paralysis or weak reinnervation, tibialis posterior muscle strength <4/5, foot drop for over 18 months, and presence of neuromuscular or metabolic disorders are the contraindications of this combined surgical approach.
SURGICAL ANATOMY
The tibial nerve, located in the posterior compartment of the leg, serves as the primary donor nerve, giving rise to branches that can be utilized for transfer, such as the soleus motor branch, medial/lateral gastrocnemius motor branches, and fascicles of the flexor digitorum longus (FDL) or flexor hallucis longus (FHL).[4,5,9] The deep peroneal nerve (DPN) is the recipient nerve for ankle dorsiflexion, innervating the tibialis anterior, extensor hallucis longus, extensor digitorum longus, and peroneus tertius muscles. The superficial peroneal nerve (SPN) serves as the recipient nerve for foot eversion and provides sensory innervation to the dorsum of the foot. In the musculoskeletal system, the tibialis posterior tendon, which originates from the posterior aspect of the tibia and fibula and inserts into the navicular and medial cuneiform bones, is the donor tendon for transfer. The tibialis anterior tendon, originating from the lateral tibia and inserted into the medial cuneiform and first metatarsal bones, is the recipient tendon for transfer. Understanding this anatomy is crucial for successfully executing the dual nerve and tendon transfer procedure, ensuring proper identification and isolation of the relevant structures, and minimizing the risk of complications.
SURGICAL TECHNIQUE
Patient positioning and anesthesia
The patient is placed in the prone position under spinal anesthesia, with a tourniquet inflated on the affected limb.
Incisions
Posterior leg incision
A curvilinear incision is made posteriorly, extending from the upper popliteal fossa to the gastrocnemius insertion, then curving toward and ending 2–3 cm below the fibula head [Figure 1]. The skin and subcutaneous tissue are retracted to expose the underlying medial and lateral gastrocnemius muscles.
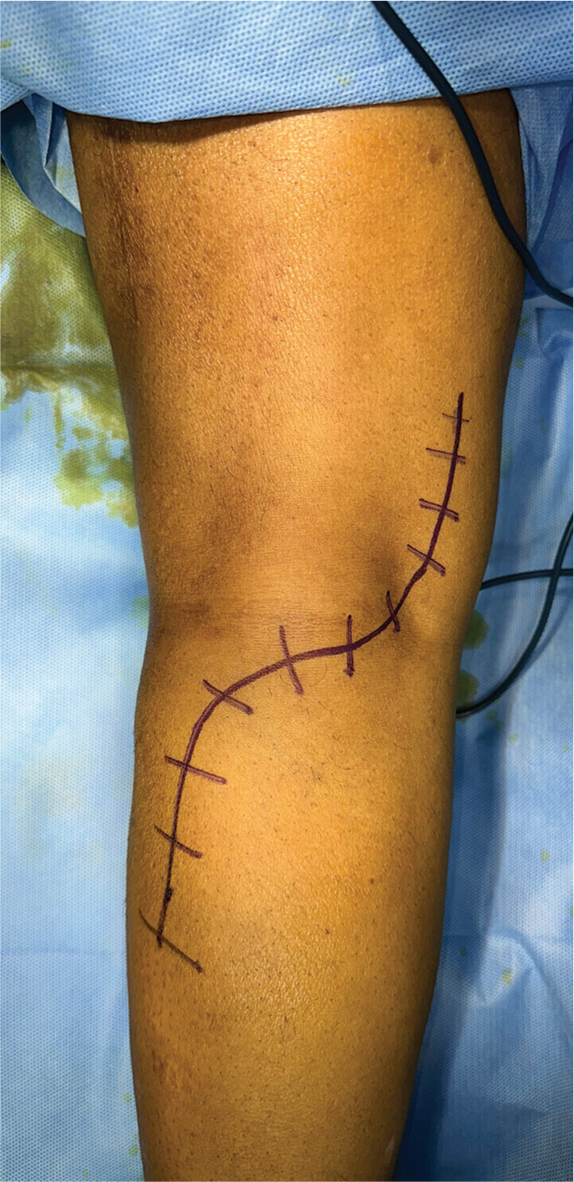
- The skin incision over the posterior aspect of the knee.
Common peroneal nerve identification and release
The common peroneal nerve is identified between the biceps femoris tendon and the lateral gastrocnemius [Figure 2]. The overlying fascia is released, allowing the nerve to be lifted and its distal course visualized. The thick fascia overlying the tibialis anterior is released to free the nerve as it enters the anterolateral compartment. The dissection continues around the fibular neck and deep into the fibularis longus, where the nerve divides into the SPN and DPN. The tibialis anterior branch of the DPN is identified and isolated for transfer. The SPN is mobilized by releasing the overlying lateral gastrocnemius muscle to ensure tension-free transfer.

- Intraoperative identification of the common peroneal nerve.
Tibial nerve identification and release
The tibial nerve is identified between the medial and lateral heads of the gastrocnemius muscle [Figure 3]. The lateral gastrocnemius motor branch, the preferred donor for the DPN, is stimulated to confirm muscle contraction. The tibial nerve is further traced distally, where it gives off branches to the popliteus, medial gastrocnemius, soleus, and plantaris muscles. At the mid-gastrocnemius level, branches to the FDL or FHL are identified as potential donors for the SPN transfer.

- Proximal identification of the tibial nerve originating from the sciatic nerve during surgery.
Fascicle separation
The epifascicular epineurium of the distal common peroneal nerve is opened, and the deep (motor) and superficial (sensory) fascicles are carefully separated. This separation is typically possible up to 70 mm proximal to the fibula head [Video 1].
Lateral gastrocnemius motor branch transfer
The distal portion of the lateral gastrocnemius motor branch is transected from the tibial nerve. The mantra is “donor distal and recipient proximal.” The tibialis anterior branch of the DPN is then meticulously dissected and transposed beneath the lateral gastrocnemius muscle. The two nerve ends are coapted using microsutures, ensuring tension-free anastomosis [Figures 4 and 5].

- The nerve to the lateral gastrocnemius is identified as the donor nerve arising from the tibial nerve.

- Intraoperative image of the lateral gastrocnemius nerve transfer to the deep peroneal nerve branch of the anterior tibial muscle.
FHL motor branch transfer
The motor branch to the FHL is carefully isolated, and its function is confirmed by electrical stimulation. The distal portion of the FHL motor branch is then transected. The SPN is mobilized by further releasing the lateral gastrocnemius muscle, and the FHL motor branch is coapted to it using microsutures and fibrin glue, again ensuring a tension-free connection [Figures 6 and 7].

- Intraoperative image of the flexor hallucis longus branch of the tibial nerve being transferred to the superficial peroneal nerve.

- Illustrative diagram of the distal nerve transfers for foot drop. Blue: Transfer of the deep peroneal branch of the anterior tibial muscle to the lateral gastrocnemius nerve branch. Green: Transfer of the superficial peroneal nerve branch to the flexor hallucis longus branch of the tibial nerve.
Tibialis posterior tendon transfer: Surgical steps
To perform tibialis posterior tendon transfer, a longitudinal incision is made over the navicular tuberosity to expose and release the tendon, which is then trimmed and secured with a non-absorbable suture [Video 1]. A second longitudinal incision along the distal posteromedial tibia exposes the proximal tendon. A subcutaneous tunnel is then meticulously created, connecting this incision to a transverse incision over the tibialis anterior tendon. The distal tibialis posterior tendon is guided through the tunnel into the anterior compartment. With the foot in neutral or slight dorsiflexion, the tendon is woven into the tibialis anterior using a Pulvertaft technique and securely sutured. Finally, all incisions are closed in layers, and a sterile dressing is applied.
Wound closure
All incisions are closed in layers with non-absorbable sutures.
Post-operative care
Postoperatively, the affected limb is immobilized in an above-knee splint for three weeks to promote initial healing and nerve coaptation. This is followed by a below-knee splint for an additional three weeks to allow gradual mobilization while protecting the surgical site. After the immobilization period, an intensive physiotherapy program is initiated, focusing on motor re-education and strengthening exercises to facilitate the recovery of ankle and foot dorsiflexion [Table 1]. Electrical stimulation of the donor nerves commences six weeks postoperatively, with six daily sessions, to encourage axonal regeneration and muscle reinnervation. This stimulation continues until clinical and/or electromyographic evidence of reinnervation is observed in the tibialis anterior and peroneal muscles.
| Phase | Duration | Goals | Interventions | Exercises |
|---|---|---|---|---|
| 1: Early protection and immobilization | 0–3 weeks | Protect surgical site, promote healing, manage edema | Above-knee splint, edema management, pain control | Isometric exercises, ankle pumps/circles (if tolerated) |
| 2: Gradual mobilization and proprioception | 4–6 weeks | Initiate movement, weight-bearing, sensory re-education | Below-knee splint/walking boot, partial weight-bearing, electrical stimulation (donor nerves) | Active/passive ROM, assisted active movements, proprioception exercises (e.g., toe curls, marble pickups, toe-to-heel rocks), gait training (parallel bars, progressing to unsupported walking) |
| 3: Strengthening and functional integration | 7–12 weeks | Increase strength/endurance, improve gait, facilitate functional activities | Full weight-bearing, continue electrical stimulation, wean off assistive devices | Progressive strengthening (e.g., resistance bands, weight cuffs), balance/coordination (e.g., single-leg stance, wobble board), exercises, functional activities training (e.g., stair climbing, uneven surfaces) |
| 4: Maintenance and optimization | Beyond 12 weeks |
Maintain gains, optimize function, prevent recurrence | Continue home exercise program, regular follow-ups | Advanced strengthening, plyometrics, activity-specific training |
ROM: Range of motion
Illustrative case
A 54-year-old male presented with a 5-month history of left foot drop following a left hemiarthroplasty. The patient had been using an ankle-foot orthosis (AFO) but found it cumbersome and uncomfortable, hindering his gait. He sought treatment for foot drop correction at our department. Under spinal anesthesia and tourniquet control, the patient was placed in a prone position. A posterior curvilinear incision was made, and the skin and subcutaneous tissues were retracted [Video 1]. The common peroneal nerve and its deep and superficial branches were identified and isolated. The tibial nerve was also exposed, and its branches, the lateral gastrocnemius motor branch and the FHL motor branch, were identified as potential donor nerves.
The DPN branch innervating the tibialis anterior was transferred to the lateral gastrocnemius motor branch, routing it beneath the lateral gastrocnemius muscle to ensure a tension-free connection. Similarly, the FHL motor branch was transferred to the SPN, also passing it beneath the lateral gastrocnemius muscle. In addition to the dual nerve transfer, a tibialis posterior tendon transfer to the tibialis anterior was performed through a separate incision in the distal ankle. The patient was then immobilized with a splint. Postoperatively, the patient underwent intensive physiotherapy, electrical stimulation, and strengthening exercises. He demonstrated immediate improvement in ankle dorsiflexion and regained grade M4 strength in both the peroneal and tibialis anterior muscles at two years of follow-up.
This case highlights the potential benefits of a combined approach to foot drop management. The tibialis posterior tendon transfer acted as an “internal splint,” providing immediate mechanical support for dorsiflexion, while the distal nerve transfers facilitated reinnervation. This dual strategy allowed for early mobilization and functional recovery, ultimately leading to a successful outcome for the patient. In the author’s series of six patients, all demonstrated improved dorsiflexion and eversion, achieving a muscle strength grade of at least medical research council 3. None of the patients required an AFO, and all reported good satisfaction, with restored strength and balance in their gait.
Pearls
The dual nerve and tendon transfer for foot drop offers a comprehensive approach by addressing both motor and sensory deficits, potentially leading to a more complete functional recovery than single interventions. This combined technique may create a synergistic effect, enhancing and accelerating the reinnervation of target muscles, resulting in improved dorsiflexion strength and function. The tendon transfer acts as an “internal brace,” providing immediate stability and support for dorsiflexion during the period of nerve regeneration.[10] Garozzo et al.[11] highlight that factors such as muscle imbalance, leading to fixed equinus deformity, can hinder nerve regeneration after peroneal nerve injury surgery. They suggest that early correction of these imbalances may improve outcomes. Inspired by this, we developed a one-stage procedure combining nerve and tendon transfers. Our findings demonstrate significant post-operative improvement and good neural regeneration at two years, supporting the efficacy of this dual approach. In addition, the nerve transfer aims to restore long-term neuromuscular control. A key advantage is the minimal donor site morbidity, as the selected nerves and tendons are typically expendable.[10]
Similarly, Lingaiah et al., research[12] reinforces the benefits of combining nerve repair with tendon transfer in common peroneal nerve injuries. Their results also showed enhanced neural regeneration and improved surgical outcomes.
Pitfalls
Despite the potential benefits, the dual nerve and tendon transfer for foot drop presents certain challenges. The procedure is technically demanding, requiring surgeons skilled in both microsurgery and tendon transfer techniques. Careful patient selection is crucial, with ideal candidates having isolated peroneal nerve injuries, adequate donor tissues, and realistic expectations. A thorough pre-operative evaluation is essential. A structured and intensive postoperative rehabilitation program is also paramount for optimizing outcomes. As with any surgery, complications such as infection, wound healing issues, nerve damage, or tendon non-union can arise, underscoring the importance of meticulous surgical technique and post-operative care.
In addition, this combined approach’s long-term efficacy and durability require further investigation, as current data are limited. The timing of surgery is also a consideration, balancing early intervention with allowing for the resolution of inflammation. By recognizing these potential pitfalls, surgeons can make informed decisions, tailor the procedure to individual needs, and provide comprehensive care to maximize patient outcomes.
DOES HARVESTING THE TIBIALIS POSTERIOR TENDON INCREASE THE RISK OF FUTURE FLATFOOT DEFORMITY AND COMPROMISE PLANTAR FLEXION?
We acknowledge the concerns about potential long-term complications associated with tibialis posterior tendon transfer, including the risk of flatfoot deformity and diminished plantar flexion strength. However, our primary goal with this combined technique is to prioritize early functional recovery and improve patient satisfaction. By utilizing the tendon as an “internal splint,” we provide immediate and dynamic support for dorsiflexion, enabling patients to regain mobility sooner and potentially reducing the psychological burden of foot drop. While the risk of long-term complications like flatfoot deformity exists, we believe careful patient selection, meticulous surgical technique, and a structured post-operative rehabilitation program can significantly mitigate these risks. The literature does report cases of flatfoot development after tibialis posterior transfer, even in patients with good functional outcomes; however, these cases rarely led to disability or required further surgery. In addition, studies indicate that the loss of plantar flexion strength is often compensated by other muscles, minimizing the impact on gait.
Our approach aims to balance the immediate benefits of early mobilization against potential long-term risks. By carefully selecting appropriate candidates and adhering to best practices in surgery and rehabilitation, we believe that we can optimize overall functional outcomes and quality of life for patients with foot drop.
DOES THE TRANSFERRED TIBIALIS POSTERIOR RETAIN FUNCTIONALITY AFTER REINNERVATION?
At the two-year follow-up, as anticipated, the primary responsibility for dorsiflexion had indeed shifted back to the reinnervated tibialis anterior and peroneal muscles, demonstrating the success of the nerve transfer. The transferred tibialis posterior tendon, having served its crucial initial role as an “internal splint” during the early recovery phase, likely assumed a less active role in dorsiflexion as the native muscles regained strength and neuromuscular control.
While the transferred tendon loses its original function as a plantar flexor, the overall impact on ankle function and gait appears minimal in our experience. This is likely due to the compensatory action of other plantar flexor muscles, such as the FDL and FHL. It is important to emphasize that the primary benefit of the tibialis posterior transfer lies not in its long-term function as a dorsiflexor, but rather in its ability to facilitate early mobilization and potentially create a more favorable environment for nerve regeneration.[13] This early intervention allows for quicker functional recovery and may contribute to improved long-term outcomes.
COMPLICATIONS
While the author has not encountered complications with this technique, it is crucial to acknowledge the inherent risks associated with any surgical intervention. Potential complications of dual nerve and tendon transfer include incomplete or delayed reinnervation, neuroma formation, and sensory disturbances related to nerve transfer. Tendon transfer complications can include tendon rupture or dehiscence, over- or under-correction leading to abnormal foot positioning, scar tissue formation limiting tendon function, and donor site morbidity.[4,5] General surgical risks should also be considered, such as infection, hematoma/seroma formation, wound healing issues, deep vein thrombosis, and anesthesia complications. Meticulous surgical technique and comprehensive post-operative care are essential to minimize these risks and maximize the success of this procedure.
DISCUSSION
Foot drop, a debilitating condition resulting from peroneal nerve injury, presents a unique challenge in reconstructive surgery. Traditional approaches, such as tendon transfers alone, often fail to achieve complete functional restoration.[4-6] While nerve transfers have shown promise in restoring motor function, they may not provide adequate initial support for ankle and foot dorsiflexion during the reinnervation process.[7,8]
This article highlights the potential benefits of a novel, integrated approach to foot drop management, combining distal tibial nerve transfers with tibialis posterior tendon transfer. This technique aims to accelerate recovery and enhance long-term functional outcomes by concurrently addressing both neural and mechanical deficits.
The strength of this approach lies in its dual mechanism of action. The tendon transfer provides immediate mechanical support (internal splint), allowing for early mobilization and preventing joint contractures. Simultaneously, the nerve transfer sets the stage for the reinnervation of the denervated muscles, potentially leading to improved muscle strength and coordination over time.
To the best of our knowledge, this combined approach has not been previously reported in the literature. Our case demonstrates this technique’s feasibility and potential efficacy in achieving significant functional improvement and patient satisfaction. Beyond peroneal nerve injuries, this combined approach holds promise for addressing foot drop in other patient populations, such as those with spinal cord injuries, spinal canal stenosis, or peripheral neuropathies. However, we recognize the need for further research to establish its efficacy and safety in these diverse clinical scenarios.
While further studies with larger patient cohorts and longer follow-ups are needed to validate these findings, our initial results suggest that this integrated approach holds promise as a valuable addition to the armamentarium for treating foot drop. By combining the strengths of both nerve and tendon transfers, we can potentially overcome the limitations of each technique and provide a more comprehensive solution for patients with this debilitating condition.
CONCLUSION
The dual nerve and tendon transfer technique is a novel approach to foot drop treatment that has the potential to improve functional outcomes for patients. By combining the benefits of both nerve and tendon transfers, this integrated approach may offer a more comprehensive solution for foot drop management. While further research is needed to fully evaluate its long-term efficacy and safety, early results suggest that this technique is a promising addition to the reconstructive surgeon’s armamentarium.
AUTHORS’ CONTRIBUTIONS
JTJJ has done the conceptualization, methodology/study design, software, validation, formal analysis, investigation, resources, data curation, writing the original draft, writing review and editing, visualization, supervision, project administration, and funding acquisition. DK has drawn all images and relevant audiovisual inputs. Both authors have critically reviewed and approved the final draft and are responsible for the manuscript’s content and similarity index.
ETHICAL APPROVAL
Ethical approval was obtained from the Ethical Committee Board of OHRC, reference number 10/2024, dated June 23, 2024
DECLARATION OF PATIENT CONSENT
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published, and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY FOR MANUSCRIPT PREPARATION
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
CONFLICTS OF INTEREST
There are no conflicting relationships or activities.
Video I available online at
FINANCIAL SUPPORT AND SPONSORSHIP
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Nerve transfers for persistent traumatic peroneal nerve palsy: The Inselspital Bern experience. Neurosurgery. 2015;77:572-9. discussion 579-80
- [CrossRef] [Google Scholar]
- Clinical results of transferring a motor branch of the tibial nerve to the deep peroneal nerve for treatment of foot drop. Neurosurgery. 2013;73:609-15. discussion 615-6
- [CrossRef] [Google Scholar]
- Management and outcomes in 353 surgically treated sciatic nerve lesions. J Neurosurg. 2004;101:8-17.
- [CrossRef] [Google Scholar]
- Tendon transfer in foot drop: A systematic review. Arch Orthop Trauma Surg. 2023;143:773-84.
- [CrossRef] [Google Scholar]
- Outcomes of the bridle procedure for the treatment of foot drop. Foot Ankle Int. 2015;36:1287-96.
- [CrossRef] [Google Scholar]
- Surgical technique of a partial tibial nerve transfer to the tibialis anterior motor branch for the treatment of peroneal nerve injury. Ann Plast Surg. 2012;69:48-53.
- [CrossRef] [Google Scholar]
- Outcomes of nerve transfers in peroneal nerve palsy. Plast Surg (Oakv). 2024;32:235-43.
- [CrossRef] [Google Scholar]
- Clinical outcomes of nerve transfers in peroneal nerve palsy: A systematic review and meta-analysis. J Reconstr Microsurg. 2019;35:57-65.
- [CrossRef] [Google Scholar]
- Surgical anatomy and exercises using the chicken thigh sciatic nerve for microsurgery training. J Hand Microsurg. 2022;15:365-70.
- [CrossRef] [Google Scholar]
- Adolf Stoffel and the development of peripheral neurosurgical reconstruction for the management of paralysis: One hundred years of nerve transfer surgery. J Musculoskelet Surg Res. 2019;3:40-6.
- [CrossRef] [Google Scholar]
- Surgical treatment of common peroneal nerve injuries: Indications and results: A series of 62 cases. J Neurosurg Sci. 2004;48:105-12.
- [Google Scholar]
- Functional evaluation of early tendon transfer for foot drop. J Orthop Surg (Hong Kong). 2018;26:2309499018799766.
- [CrossRef] [Google Scholar]
- Long-term results of tibialis posterior tendon transfer for drop-foot. Int Orthop. 2001;25:114-8.
- [CrossRef] [Google Scholar]






