Translate this page into:
Effectiveness of autologous fat graft and methylprednisolone as single and combination therapy in the prevention of epidural fibrosis
*Corresponding author: Andhika Yudistira, Department of Orthopaedic and Traumatology, Faculty of Medicine Universitas Brawijaya - Dr. Saiful Anwar General Hospital, Malang, East Java, Indonesia. andhika.yudhistira@ub.ac.id
-
Received: ,
Accepted: ,
How to cite this article: Yudistira A, Asmiragani S, Satriawan E, Aristoteles A, Kristanto H. Effectiveness of autologous fat graft and methylprednisolone as single and combination therapy in the prevention of epidural fibrosis. J Musculoskelet Surg Res. 2025;9:225-9. doi: 10.25259/JMSR_418_2024
Abstract
Objectives:
Epidural fibrosis is a common complication following laminectomy, contributing to failed back surgery syndrome characterized by back pain and radiculopathy. Despite ongoing research, no consensus exists on the best method to prevent epidural fibrosis. This animal study aimed to compare the effectiveness of autologous fat grafts and methylprednisolone as single and combination therapies in preventing epidural fibrosis.
Methods:
Four groups of rats, each containing 7, underwent different treatments post-lumbar laminectomy: A control group, a group receiving methylprednisolone in the epidural space, a group receiving an autologous fat graft, and a group receiving both methylprednisolone and an autologous fat graft. After 6 weeks, the formation of epidural fibrosis was evaluated through histopathological examination based on the classification. The degree of epidural fibrosis was analyzed.
Results:
The autologous fat graft group exhibited the lowest degree of fibrosis (Grade 1). Comparisons between this group and the others showed a significant difference (P < 0.05). No significant difference was observed between the control, methylprednisolone, and combination groups (P > 0.05).
Conclusion:
The study demonstrated a significant reduction in histological epidural fibrosis using autologous fat grafts compared to methylprednisolone and their combination in animal models. Histological analysis indicated that autologous fat grafts resulted in a lower degree of fibrosis following laminectomy.
Keywords
Autograft
Fibrosis
Laminectomy
Methylprednisolone
Rattus norvegicus
INTRODUCTION
Epidural fibrosis is a complication that can arise after laminectomy, leading to Failed Back Surgery Syndrome (FBSS).[1] FBSS affects 8–40% of patients after lumbar laminectomy. It is diagnosed when patients continue to experience troublesome or recurring back or leg pain after spinal surgery. Similarly, patients with cervical surgery may continue to feel neck and arm pain or myelopathy symptoms.[2]
The main causes of back and leg pain in epidural fibrosis are the spread of fibrous tissue into the neural canal and adhesion to the dura mater. Fibrous tissue formation has three phases: An inflammatory reaction 3–5 days post-surgery, involving coagulation and chemokine release; a second phase, 2–3 weeks later, with fibroblast proliferation regulated by transforming growth factor-P1, interleukin-6, and fibroblast growth factor; and a reconstruction phase over months to years with connective tissue deposition around the lesion.[2]
Epidural fibrosis is found in 24% of FBSS patients, with post-Hernia Nucleus Pulposus surgery incidences ranging from 1% to 48%. Studies have shown that extensive epidural fibrosis on magnetic resonance imaging (MRI) correlates with increased back pain and recurring radicular pain. Normal wound healing after lumbar surgery replaces epidural fat with fibrotic tissue, which excessive bleeding, root traction, anatomical anomalies, and tissue reactions to foreign bodies can exacerbate.[3]
Reoperation increases epidural fibrosis to 60%, worsening the prognosis. Compression from fibrosis makes nerve roots more susceptible to damage and increases the risk of tearing the dural layer.[4] One treatment option is fat tissue transplantation after microdiscectomy, but its effectiveness is debated. Dobran et al.[5] found no link between fat transplantation and reduced epidural fibrosis, while Bryant et al.[6] noted increased revascularization. Görgülü et al. concluded that it was ineffective.[7,8]
Methylprednisolone, a synthetic glucocorticoid, has anti-inflammatory, anti-allergic, and immunosuppressive properties. Steroids inhibit cell migration to inflamed areas, cytokine synthesis, and fibroblast growth. A study on long-acting steroids for preventing epidural fibrosis showed that dexamethasone irrigation during laminectomy significantly reduced hospital stays and narcotic use by inhibiting inflammation and pain mediators, and it lowered fibrosis rates.[9]
No scientific study has been conducted on the effectiveness of combining autologous fat grafts with steroids to prevent epidural fibrosis. Therefore, this animal study aimed to compare the effectiveness of autologous fat grafts and methylprednisolone as single and combination therapies in preventing epidural fibrosis.
MATERIALS AND METHODS
Study design
The research design used is a randomized, post-test-only, and controlled group design. This experimental laboratory study, which focuses on the degree of epidural fibrosis using a post-test-only control group design, was conducted at the Biomedical Laboratory of the Faculty of Medicine, Universitas Brawijaya, from August to December 2022.
Sample size
We applied the Federer formula to determine the sample size in this experimental work. Federer’s formula is (n-1) (t-1) ≥ 15, where n represents the sample size of each group and t is the number of groups. This study has four groups, and on following the Federer formula, it was determined that each group requires a sample size of at least six. To pre-emptively address issues in experimental animals, we deliberately increased the sample size to 10 per group. In the initial study, 40 rats survived until the end of the treatment period (6 weeks), with 10 rats in each group. After termination and histopathological examination, 28 samples were found eligible for the study, as 12 other samples still contained remnants of the lamina under microscopic examination. Therefore, we used only 28 rats for this research.
Sampling method
The study involved male Wistar albino rats (Rattus norvegicus) aged 11–12 weeks and weighing 300–400 g. Four groups of rats, each containing seven rats, were used (Group I as control, Group II with fat graft, Group III with methylprednisolone, and Group IV with a combination of fat graft and methylprednisolone), totaling 28 rats. Sampling was done through purposive random sampling followed by simple randomization to select rats for each group. Rats showing signs of illness (such as dull or shedding fur, loose skin, abnormal eyes, feces, behaviors, or breathing patterns) were excluded from the study. The samples were divided into four groups using simple randomization to distribute uncontrollable confounding variables across all groups equally.
Procedure
All rats received a single prophylactic dose of ceftriaxone (50 mg/kg body weight) intramuscularly 30 min before surgery. General anesthesia was induced using intramuscular ketamine hydrochloride (30 mg/kg body weight), and the rats were placed in the prone position. The surgical field was prepared with shaving, washing, disinfection, and antiseptic treatment with povidone-iodine. Sterile drapes were used for draping. A 3 cm midline posterior incision was made to expose the spinous processes and lumbar vertebral segments. Total laminectomy of L4-5-6 and excision of the ligamentum flavum were performed to expose the dura mater and nerve roots. Fat grafts were administered in groups 2 and 4 into the epidural space, and methylprednisolone (10 mg/kg body weight) was administered locally into the epidural space in groups 3 and 4.
All rats were euthanized after 6 weeks (42 days) using high-dose sodium thiopental (75–100 mg/kg body weight). The lumbar vertebral column was harvested en bloc with osteotomy, and histopathological preparations were made. Rats with dural tears, nerve root injuries, post-operative neurological deficits, or infections were excluded from the study. The extent of fibrosis in the laminectomy area and its relationship with the dura mater were evaluated based on histological criteria and classifications found in the literature. Comparisons were made within groups and with the control group, and the results were statistically analyzed. Epidural fibrosis was assessed using the He et al. scale, where scale 0 means no fibrosis in the dura mater, scale 1 indicates the presence of fibrosis between scar tissue and the dura mater, scale 2 indicates adhesion in two-thirds of the laminectomy area, and scale 3 indicates adhesion extending beyond two-thirds of the laminectomy area.[10]
Statistical analysis
The comparative hypothesis test steps included a normality test using the Shapiro–Wilk test, followed by comparative testing with the Mann–Whitney test. Data analysis was performed using IBM Statistical Package for the Social Sciences Statistics version 27, with a significance level of 0.05 (P = 0.05) and a confidence level of 95% (α = 0.05).
RESULTS
Figure 1 shows normal lumbar vertebrae. Histopathological epidural fibrosis is shown in Figures 2 and 3. Table 1 shows a summary of fibrosis grading across all samples.
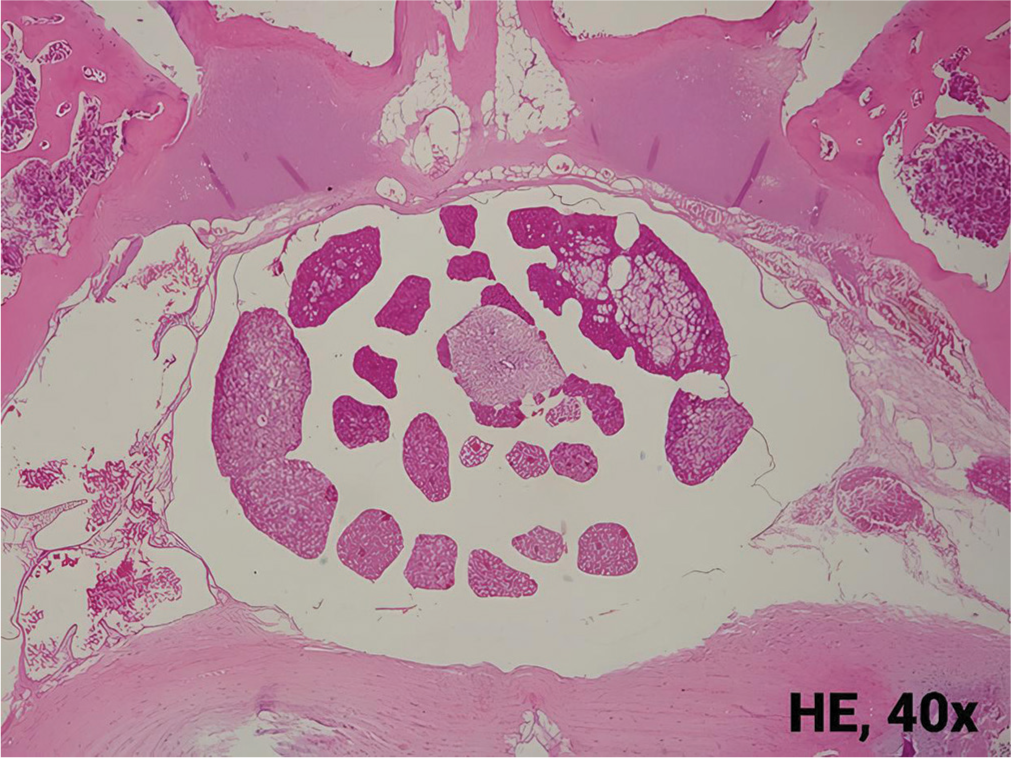
- Axial view of normal lumbar vertebrae. HE: Hematoxylin and eosin.
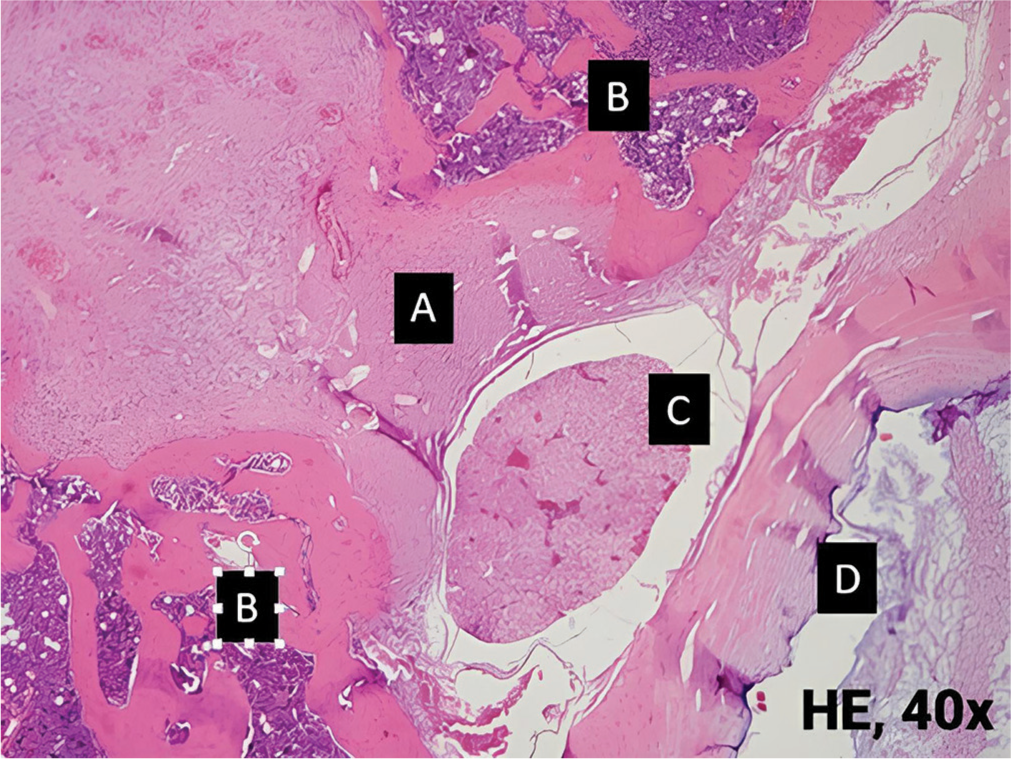
- Histopathological view of grade III epidural fibrosis on Combination of Methylprednisolone and Fat group. (A): Epidural fibrosis, (B): Bone, (C): Cauda equina, (D): Vertebral body. HE: Hematoxylin and eosin.
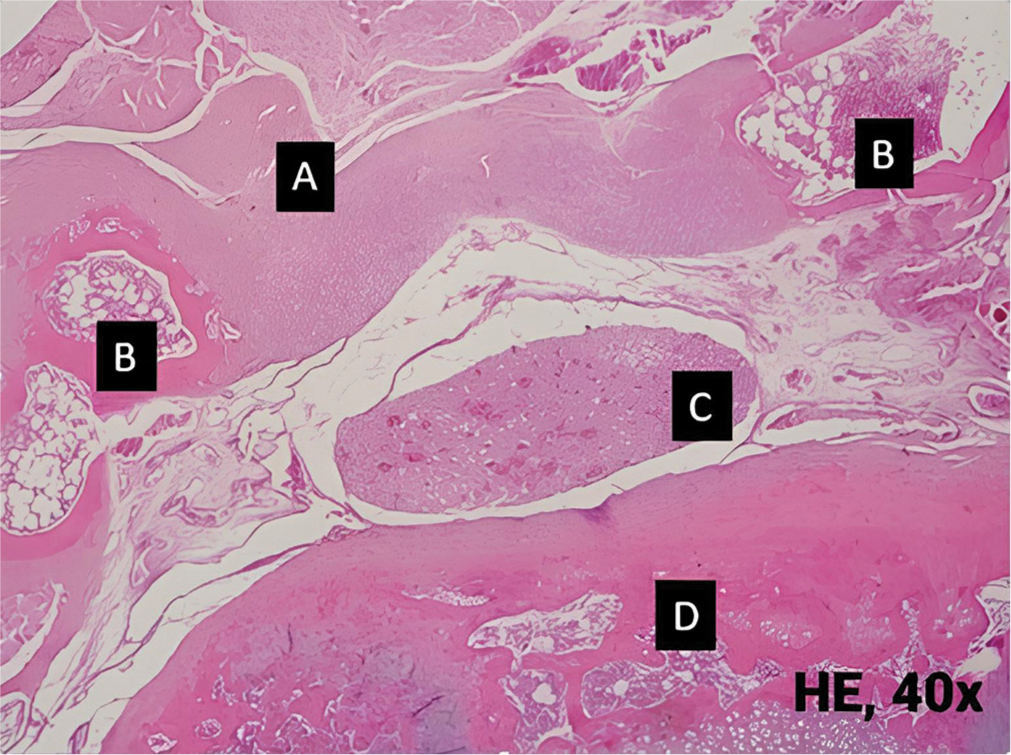
- Histopathological view of grade I epidural fibrosis on Autologous fat group. Only slight fibrous tissue is observed. (A): Epidural fibrosis, (B): Bone, (C): Cauda equina, (D): Vertebral body. HE: Hematoxylin and eosin.
| Groups | Number of samples (n) | Fibrosis grading |
|---|---|---|
| Control | 7 | 3 |
| Methylprednisolone | 7 | 3 |
| Autologous fat graft | 7 | 1 |
| Combination of methylprednisolone+Autologous fat graft | 7 | 3 |
Based on the Shapiro–Wilk test, the significance value was 0.0000. In the normality test, if the significance <0.05, the data were not normally distributed. Therefore, a non-parametric analysis of the Mann–Whitney test was then used.
The Mann–Whitney test results indicated that the degree of epidural fibrosis showed a significant difference in the mean values of the groups if it had P < 0.05. The results of the Mann–Whitney test for each group are shown in Table 2.
| Group comparison | P-value | Interpretation |
|---|---|---|
| Control | ||
| Methylprednisolone | 1.000 | Insignificant |
| Fat | 0.001 | Significant |
| Methylprednisolone+Fat | 1.000 | Insignificant |
| Methylprednisolone | ||
| Fat | 0.003 | Significant |
| Methylprednisolone+Fat | 1.000 | Insignificant |
| Fat | ||
| Methylprednisolone+Fat | 0.000 | Significant |
The Mann–Whitney test results for epidural fibrosis grade showed that the fat group had the lowest fibrosis grade, Grade 1. The comparison between the fat group and the other groups (Control, Methylprednisolone, and Methylprednisolone + Fat) showed a significant difference (P < 0.05). However, the comparison between the Control, Methylprednisolone, and Methylprednisolone+Fat groups showed no significant difference (P > 0.05).
DISCUSSION
This study demonstrated that rats receiving autologous fat pads in the laminectomy defect area showed histological evidence of thinner fibrosis compared to those treated with methylprednisolone alone or a combination of methylprednisolone and fat. A descriptive analysis of treatments among fat, methylprednisolone, and their combination was conducted, with results tested using the Mann–Whitney test, revealing significant differences in epidural fibrosis degrees between groups (P < 0.05).
Corticosteroids, often used as anti-inflammatory drugs, can block Cyclooxygenase-2 messenger ribonucleic acid, thereby suppressing the production of prostaglandins, ILs, and other inflammatory mediators, reducing pain. Studies have shown that methylprednisolone, a long-acting steroid, interacts extensively with nerve tissue and distributes widely on the spinal cord surface. Steroids inhibit cell migration to inflamed areas, synthesis of acute-phase reactants, cytokine synthesis and release, and further infiltration of neutrophils, monocytes, or macrophages at the inflammation site, reducing fibroblast infiltration in the tissue.[9]
The use of topical steroids in rats did not significantly reduce epidural fibrosis compared to a combination of steroids and gelatine sponge. Tian observed fibroblast and inflammatory cell hyperplasia in rats treated with topical steroids four weeks post-laminectomy, with increased fibroblast numbers and extensive adhesion between the dura mater and fibrous tissue at eight weeks.[11]
In 2020, Guler et al. compared the effects of platelet-rich plasma, fat graft, and collagen matrix in rat experiments, finding no allergic reactions or infections.[7] Histological examination of the fat graft group showed Grade 1 fibrosis in 71% of samples and Grade 2 fibrosis in the remaining samples. Revascularization of less than two-thirds of the lamina length was observed, indicating extensive fibrotic tissue. This study demonstrated that fat grafts performed better clinically in post-operative conditions and showed lower epidural fibrosis on contrast MRI.[12,13]
Free fat transfer, an autogenous material, is easily obtained without the risk of foreign body reactions. Initially, it was believed that fat tissue prevented local scar tissue formation, as observed by surgeons. However, using free fat transfer as effective prophylaxis in spine surgery remains debated, as fat tissue does not increase or change when filling the defect.[14]
Histopathological confirmation shows that free fat transfer grafts do not eliminate local adhesions or fibrous formation between root areas or the thecal sac. However, due to their lack of foreign body reactions, these materials can form isolation layers between anatomical structures without causing mass effects or adhesions.[14]
Notable limitations of this study include using small animal models, which may result in higher mortality rates under stress. Using animals closer to humans in a lineage is preferred to predict human responses more accurately. The high mortality rate suggests that a larger sample size may be necessary for future studies.
CONCLUSION
Based on the results and discussion of this study, significant differences were observed in the histological grading of epidural fibrosis using the He grading scale in post-laminectomy animal models. Specifically, autologous fat grafts were compared to methylprednisolone and a combination of both treatments. The findings revealed that autologous fat grafts produced a notably different degree of epidural fibrosis than other treatments.
Moreover, the study found that using autologous fat grafts resulted in a lower degree of fibrosis histologically, according to the He grading scale, in laminectomy defects of animal models. This suggests that autologous fat grafts may be more effective in reducing fibrotic tissue formation following laminectomy than methylprednisolone or a combination of both treatments.
Recommendation
In light of these findings, further research is recommended to explore using a combination of physical barrier materials and other active drug substances. This approach could potentially enhance the effectiveness of treatments aimed at preventing epidural fibrosis post-laminectomy, providing better outcomes for patients undergoing spinal surgery.
Authors’ contributions
AY: Concepts, Design, Definition of Intellectual Content, Manuscript Preparation, and Data Analysis. SA and ES: Concepts, Definition of Intellectual Content, Manuscript Editing, and Review. AA: Data Acquisition, Experimental Studies, and Manuscript Preparation. HK: Experimental Studies, Manuscript Preparation, and Statistical Analysis. All authors have critically reviewed and approved the final draft and are responsible for the manuscript’s content and similarity index.
Ethical approval
The research/study approved by the Institutional Review Board at Faculty of Medicine Universitas Brawijaya, Malang, East Java, Indonesia, number 70B/EC/KEPK-PPDS/06/2022, dated June 15, 2022.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that AI technology was not used to assist in writing or editing the manuscript, and no images were manipulated using AI.
Conflicts of interest
There are no conflicting relationships or activities.
Financial support and sponsorship: This study received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Interrater reliability of the postoperative epidural fibrosis classification: A histopathologic study in the rat model. Asian Spine J. 2015;9:587-94.
- [CrossRef] [PubMed] [Google Scholar]
- Update on biomaterials for prevention of epidural adhesion after lumbar laminectomy. J Orthop Translat. 2018;13:41-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of sodium hyaluronate and methylprednisolone alone or in combination in preventing epidural fibrosis. Neurol Res. 2013;35:851-6.
- [CrossRef] [PubMed] [Google Scholar]
- Epidural fibrosis after lumbar disc surgery: Prevention and outcome evaluation. Asian Spine J. 2015;9:370-85.
- [CrossRef] [PubMed] [Google Scholar]
- Epidural scarring after lumbar disc surgery: Equivalent scarring with/without free autologous fat grafts. Surg Neurol Int. 2017;8:169.
- [CrossRef] [PubMed] [Google Scholar]
- Autogeneic fat transplants in the epidural space in routine lumbar spine surgery. Neurosurgery. 1983;13:367-70.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of platelet-rich plasma, fat pad and dural matrix in preventing epidural fibrosis. Acta Ortop Bras. 2020;28:31-5.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of epidural free fat graft on the outcome of lumbar disc surgery. Neurosurg Rev. 2004;27:181-4.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of curcumin and methylprednisolone in the prevention of epidural fibrosis after spinal surgery: An experimental study. J Exp Clin Med. 2021;38:88-93.
- [CrossRef] [Google Scholar]
- A quantitative model of post-laminectomy scar formation. Effects of a nonsteroidal anti-inflammatory drug. Spine (Phila Pa 1976). 1995;20:557-63. discussion 579-80
- [CrossRef] [PubMed] [Google Scholar]
- Preventive effect of dexamethasone gelatin sponge on the lumbosacral epidural adhesion. Int J Clin Exp Med. 2015;8:5478-84.
- [Google Scholar]
- Prospective randomized comparative study to evaluate epidural fibrosis and surgical outcome in patients undergoing lumbar laminectomy with epidural autologous free fat graft or gelfoam: A preliminary study. Int J Appl Basic Med Res. 2018;8:71-5.
- [CrossRef] [PubMed] [Google Scholar]
- Postoperative epidural fibrosis prevention: Which is better-autologous fat versus gelfoam. Asian Spine J. 2022;16:343-51.
- [CrossRef] [PubMed] [Google Scholar]
- Free autologous fat in spine surgery to prevent adhesion, cicatrisation, and cerebrospinal leakage. Assessment of the practical side of this method. Med Stud. 2020;36:224-9.
- [CrossRef] [Google Scholar]







