Translate this page into:
High-Resolution ultrasound of knee osteoarthritis in a Southwest Nigerian population: Our experience
2 Department of Traumatology, Obafemi Awolowo University, Ile-Ife, Nigeria
Corresponding Author:
Bolanle O Ibitoye
Department of Radiology, Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife
Nigeria
bobitoye@yahoo.com
| How to cite this article: Oyamakinde SO, Ibitoye BO, Esan O, Famurewa OC, Aderibigbe AS. High-Resolution ultrasound of knee osteoarthritis in a Southwest Nigerian population: Our experience. J Musculoskelet Surg Res 2019;3:209-215 |
Abstract
Objective: High-resolution musculoskeletal ultrasound (MSKUS) is more sensitive than conventional radiography (CR) in evaluating early knee osteoarthritis (OA). The aim of this study is to determine the US findings in knee OA and also to compare these with CR, which is the gold standard. Methods: One hundred and twenty patients with primary knee OA and 120 controls were examined. They all had knee US. CR included weight-bearing anterioposterior and lateral knee radiographs. Kellgren and Lawrence (K–L) grades were assessed, and tibiofemoral joint space width was measured. Data were analyzed using SPSS Version 20. Results: Two hundred and fifteen knees had OA. US findings were tibial osteophytes (67.4%), femoral osteophytes (66.5%), effusion (61.4%), synovitis (46%), medial meniscal protrusion (38.2%), Baker's cysts (34.4%), and lateral meniscal protrusion (27.5%). US detected femoral osteophytes in 13 knees that were not detected in CR. CR detected femoral osteophytes in 12 knees, which US could not. Agreement between US and CR was 74% (P < 0.001). US detected tibial osteophytes in 10 knees which CR film could not. CR detected tibial osteophytes in 9 knees, which the US could not. Agreement between US and CR was 80% (P < 0.001). Sensitivity and specificity of US in detecting femoral and tibial osteophytes were 91.6% and 82.2% for the femur and 93.8% and 85.9% for the tibia, respectively. Student's t-test showed a significant difference (P < 0.001) between femoral condylar thickness in OA patients and controls. Conclusion: MSKUS complements CR in evaluating knee OA.
Introduction
Osteoarthritis (OA) is a chronic, debilitating joint disease characterized by degenerative changes in the bones, cartilage, menisci, ligaments, and synovial tissues[1],[2] and is therefore considered a disease of the whole joint.[2],[3],[4],[5],[6] It is the most common disease of the joints[3],[7] and is considered the most common chronic disease of the elderly,[6],[7] causing pain and disability which significantly affect the quality of life.[2],[8] The knee is one of the most common joints involved in OA[7] because it is a weight-bearing joint.[2] Zhou et al.[9] stated that more than 75% of individuals over 65 years old have OA. In the Western world, the prevalence of knee OA is 9% among 20-year-old individuals, increasing to 30% in those above 60 years, and then to 90% in those between 70 and 74 years.[2] In South Africa, the prevalence of OA ranged from 29.5% in rural settings to 55.1% in urban settings and up to 82.7% in adults over 65 years.[10] In Northeastern Nigeria, the prevalence of OA was found to be 16.3% in individuals aged 30 years and above while those from 40 years had a prevalence of 20.6%.[11] Another study in Southwestern Nigeria showed that one out of four people in the adult population had OA, with a female preponderance.[1]
The diagnosis of OA is usually established late when the disease has progressed significantly and very little help can be obtained from the use of disease-modifying drugs.[12] This late presentation is partly due to the pathophysiology of OA which is complex[2] and majorly driven by articular cartilage.[10],[12] It lacks its own vascular supply and is deficient of innervations. Degenerative changes in articular cartilage, therefore, do not produce symptoms.[12] Some studies[3],[5],[12],[13] have however shown the involvement of synovitis in the pathophysiology of both early and late OA, offering a potential target for treatment of both symptoms and potential structure modification.
OA is characterized by cartilage loss, subchondral bone changes, synovial inflammation, and meniscal degeneration.[3],[4],[14] A link between synovial inflammation and progression to structural damage has been established by several studies.[4],[13],[14],[15],[16],[17]
Conventional radiography (CR) is considered the gold standard for examining the osteoarthritic knee joint.[6],[7],[8] It is the most common,[7],[8] easiest, and relatively cheapest radiological modality for diagnosis and follow-up of OA.[2],[3] The evaluation of joint width space on CR is the gold standard and has been recommended as the best method for assessment of progression of joint damage due to OA.[2],[5],[7] It is however limited in its ability to detect early cartilage changes which indisputably occur before the reduction in joint space.[5],[7],[8],[18] By the time the first knee joint changes are seen on CR, 10% of cartilage is already lost.[3] Clinically significant changes are often not apparent on CR for at least 1 or even 2 years.[6] It mainly reflects the pathologies of bone at an advanced stage,[8] detecting secondary changes such as osteophyte formation, cartilage loss and meniscal extrusion. These are what manifest on CR as joint space narrowing.[6] Its usefulness in the early detection of OA is therefore limited.[18],[19]
Newer imaging modalities such as magnetic resonance imaging (MRI) and high-frequency musculoskeletal ultrasound (MSKUS) offer a more detailed overall assessment of the osteoarthritic joint.[2] MRI is sensitive in detecting preradiographic OA in the early stages of the disease as it has the advantage of assessing the soft-tissue structures of the joint.[5] It also offers excellent soft-tissue contrast and ability to acquire morphological and biochemical data.[6] US plays a minor role in routine clinical and scientific settings but is invaluable in the assessment of inflammatory conditions in acute OA of the knee, periarticular swellings, and effusions.[2] It, however, has the advantage of being less expensive, has a shorter examination time, has good patient acceptability, and is more accessible than MRI,[7] especially in resource-poor environments. In addition, some authors have pointed out that MRI may overestimate cartilage thickness,[7],[20] unlike US, which has a good histological correlation.[4],[7] Doppler US is usually sensitive in assessing inflammatory joint conditions such as synovitis and periarticular inflammatory disease.[2],[4],[21] Contrast-enhanced US is also more sensitive than contrast-enhanced MRI in detecting synovitis.[3] However, US has the disadvantage of being operator dependent[3],[6] and is only able to assess superficial structures adequately.[3]
This study aims to report the pattern of ultrasonographic findings in knee OA, comparing these with CR.
Materials and Methods
This was a prospective, case–controlled, cross-sectional study. It was carried out at Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria. One hundred and twenty consecutive patients with unilateral or bilateral primary OA according to American College of Rheumatology standards[9] were recruited from the general outpatient department and the orthopedic clinic of the hospital between January and December 2016. The study was conducted at the Radiology Department of the hospital. Written informed consent was obtained from all patients.
Controls were age, sex, and weight-matched individuals who presented in the department for other radiological investigations not related to the knee and who did not have knee pain. Volunteers who had no joint pain in the knees and no positive history of knee disease were also recruited as controls.
Inclusion criteria were all patients referred for CR on account of pain in the knees and those with a clinical diagnosis of chronic knee OA. Exclusion criteria were patients with a clinical history of mechanical knee derangement, inflammatory arthritis, microcrystalline arthropathy, knee trauma or surgery, and patients who had received arthrocentesis and/or an intra-articular steroid injection within the last 3 months.
All the study participants underwent MSKUS of both knees using an US real-time scanner (MINDRAY DC-7) with a multifrequency linear transducer (7–12 MHz). US was performed with the patient lying supine, the knee flexed to 20–30° and a pillow under the knee for comfort. After applying scanning gel to the suprapatellar region, scanning was commenced in longitudinal, transverse, and coronal planes from medial to lateral or lateral to medial positions. Scanning of the quadriceps tendon, suprapatellar and prepatellar bursae were performed. In the infrapatellar region, the patellar tendon and infrapatellar superficial and deep bursae were scanned longitudinally and transversely. The medial collateral ligament and medial meniscus were scanned with the knee externally rotated and the patient supine. The lateral collateral ligament and lateral meniscus were scanned in the lateral aspect of the knee. The posterior aspect of the knee was scanned longitudinally and transversely with the patients in the prone position. The gastrocnemius-semimembranosus bursa, posterior meniscal horns and posterior cruciate ligaments were also scanned.[7],[8],[18],[19],[22],[23] Only the medial and lateral femoral cartilage thickness were assessed and measured in the controls.
All disease processes were identified according to standard descriptions.[8],[12],[13],[14],[24] Synovial hypertrophy and effusion had the same grading system. Grade 0 was when synovial thickness/fluid depth was <4 mm, Grade 1 when the thickness/depth was 4≤8 mm, Grade 2 when the thickness/depth was 8 mm to <11 mm, and Grade 3 ≥11 mm. Meniscal protrusion was present when the distance between the peripheral border of the meniscus and the outline of the tibial plateau measured >3 mm.[25],[26] The distance was graded as Grade 0 (<3 mm), Grade 1 (3≤5 mm), Grade 2 (5≤8 mm), and Grade 3 (≥8 mm). Synovitis was defined as an abnormal hypoechoic or anechoic intra-articular tissue that was nondisplaceable, poorly compressible, and sometimes exhibiting Doppler signal.[27],[28]
Weight-bearing anteroposterior (AP) and lateral plain knee radiographs were taken for each patient and interpreted by the same researcher who assessed the severity of OA using the modified Kellgren and Lawrence (K–L) scale.[29]
Statistical analysis was performed using the Statistical Package for the Social Science for Windows (SPSS Inc., Chicago, IL, USA), version 20. The independent Student's t-test was used to compare means between parametric variables. P < 0.05 was considered statistically significant.
Results
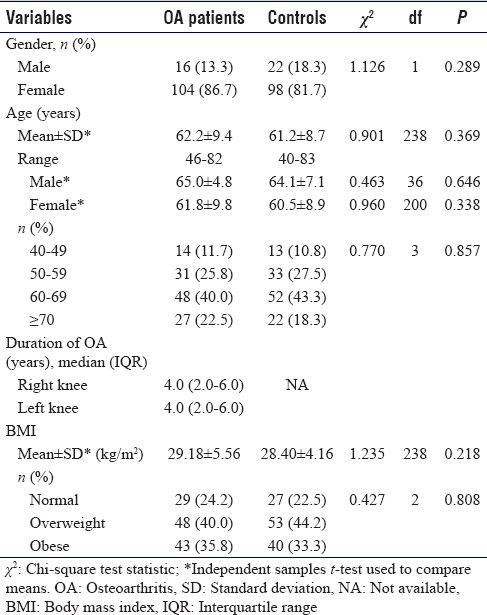
Two hundred and forty participants comprising 120 OA patients and 120 controls were recruited for this study. The mean age for OA groups and controls was 62. 2 ± 9. 4 and 61.2 ± 8.7 years, respectively. There were more females than males in the OA group, with 133% males and 86.7% females. The patients in both groups were weight-matched. In the OA group, 24.2% patients had a normal body mass index (BMI) while 22.5% had a normal BMI in the control group. Other details of the demographic data for both groups are shown in [Table - 1].
US findings in OA knees were effusion in 132 knees (61.4%) (P < 0.001), synovitis in 99 knees (46%), medial meniscal protrusion in 82 knees (38.2%) (P = 0.002), and lateral meniscal protrusion in 59 knees (27.5%) (P = 0.028), [Figure - 1]. Femoral and tibial osteophytes were seen in 143 knees (66.5%) and 145 knees (67.4%), respectively (P < 0.001). Baker's cysts were demonstrated in 74 knees (34.4%) (P < 0.001). The mean thickness for medial femoral condylar cartilage was 1.59 ± 0.71 mm while the lateral femoral condylar cartilage measured 1.79 ± 0.79 mm. Details are shown in [Table - 2].
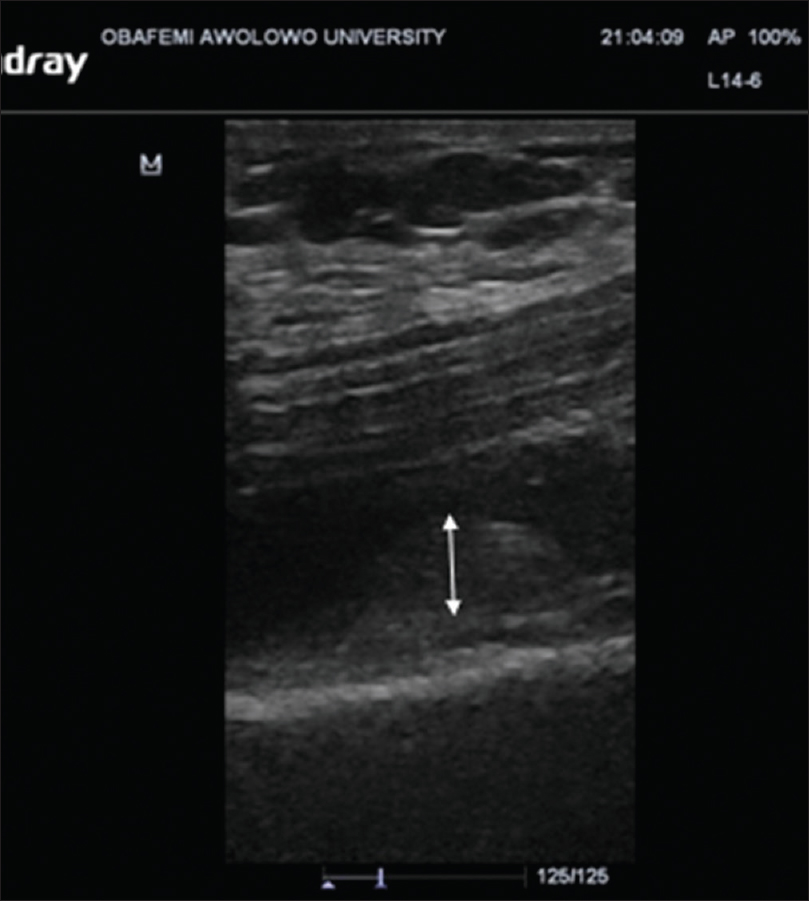 |
| Figure 1: Ultrasound image (Longitudinal plane) through the suprapatellar synovial recess showing hypertrophied synovium (double arrow) |
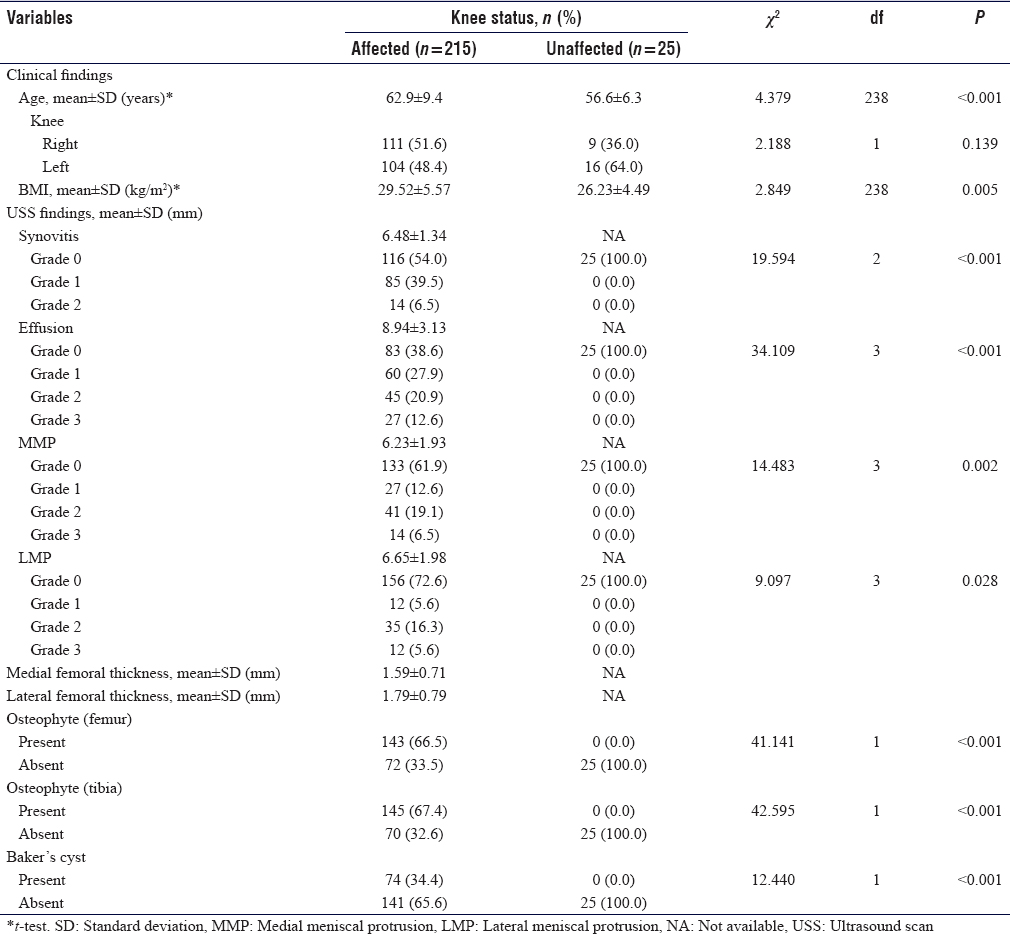
CR showed tibiofemoral degenerative changes in all OA knees. K–L Grade I was seen in 22 knees (10.2%), Grade II in 60 knees (27.9%), while Grades III and IV were seen in 70 knees (32.6%) and 63 knees (29.3%) respectively [Figure - 2]. The mean value of tibiofemoral space width for the medial compartment was 1.62 ± 1.58 mm while that of the lateral compartment was 3.39 ± 1.68 mm. Femoral and tibial osteophytes were seen in 142 knees (66%) and 144 knees (67%) (P < 0.001), respectively [Table - 3] and [Figure - 3].
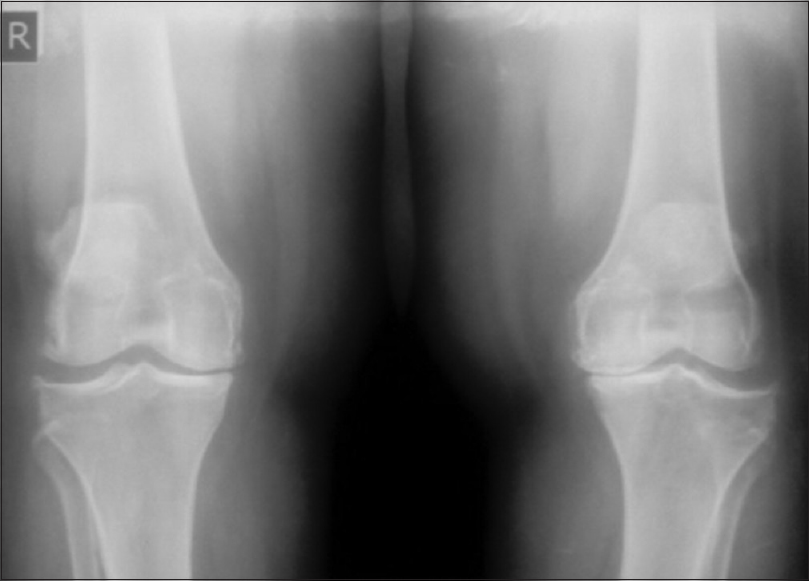 |
| Figure 2: Anteroposterior radiograph of Kellgren-Lawrence Grade 3 knees showing bilateral joint space narrowing of the medial tibiofemoral compartments and tibiofemoral bilateral osteophyte formations |
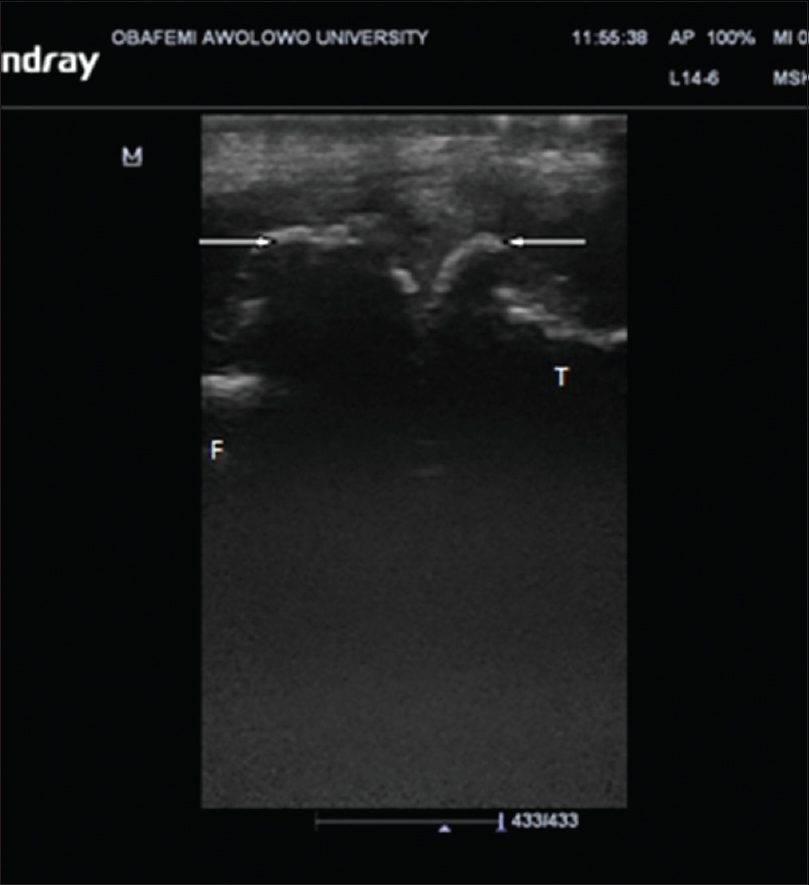 |
| Figure 3: Ultrasound (Longitudinal plane) of the medial aspect of the knee joint showing femoral and tibial osteophytes (arrows) |
US detected femoral osteophytes in 13 knees which CR was unable to detect, while CR detected femoral osteophytes in 12 knees which the US could not detect. Kappa value between US and CR was 75% (P < 0.001). Using CR as the gold standard, US sensitivity to detect femoral osteophytes was 91.6% while specificity was 82.2%.
US detected ten tibial osteophytes which CR was unable to detect while CR detected nine tibial osteophytes which US was unable to detect. Kappa value was 80% (P < 0.001). Using CR as the gold standard, US sensitivity to detect tibial osteophytes was 93.8% while the specificity was 85.9%.
Mean measurements of femoral cartilage thickness of both medial and lateral condyles in OA and control groups showed statistical significance when compared using Student's t-test (P < 0.001). On the right knee, the femoral cartilage thickness of the medial condyle for OA and control groups measured 1.58 ± 0.75 mm and 2.29 ± 0.27 mm respectively (P < 0.001). For the lateral condyle, it was 1.89 ± 0.85 mm in OA patients and 2.24 ± 0.19 in controls (P < 0.001). On the left knee, femoral cartilage thickness of the medial condyle was 1.59 ± 0.68 mm in the OA group compared to 2.27 ± 0.25 mm in healthy controls (P < 0.001) while the femoral cartilage thickness (mean ± standard deviation) of the lateral condyle for OA patients and controls measured 1.69 ± 0.70 mm and 2.23 ± 0.17 mm (P < 0.001), respectively [Table - 4].
Discussion
The use of high-resolution MSKUS in rheumatic diseases is gaining popularity,[7],[8],[19] and the availability of high-frequency transducers even in resource-poor countries like Nigeria is increasing. This allows for better visualization and therefore evaluation of superficial MSK structures. High-resolution US is in increasing demand because it is noninvasive, safe, cheap, readily available, can be easily repeated, and does not require the use of ionizing radiation.[3],[4],[19] It is more popular than MRI in our environment which is more expensive, more time-consuming,[3] and not easily available.[7]
In our study population, the most common US finding was osteophytes. Tibial and femoral osteophytes were seen in 145 (67.4%) and 143 knees (66.5%) respectively. This is possibly because most OA patients recruited into this study had K–L radiographic grades II-IV. Late presentation is a frequent occurrence in our environment, when articular cartilaginous changes, a sign of early OA detected by US, are no longer detectable. These changes cannot be visualized on plain radiographs.[4],[5],[7],[8],[19] Our findings are similar to those of Gaafar et al.[30] and Wu et al.,[31] who carried out their studies on populations similar to ours. They both found that the most common US finding in patients with symptomatic OA was osteophytes. The prevalence of osteophytes in their studies was 100% and 88%, respectively. The higher prevalence rate recorded in Gaafar et al.'s study[30] may be due to their smaller sample size, as only 15 patients with knee OA were examined, while in the study by Wu et al.,[31] OA patients with equal radiographic scales were used and 92% of the population group were in K–L Stages III and IV.
The US findings of knee OA in this study [Table - 2] were in keeping with other studies.[25],[30]
Radiographic scoring of joint damage relies mainly on joint space narrowing and osteophytes, both of which take some time to manifest. Studies have found a moderate correlation between radiographic joint space narrowing and loss of hyaline articular cartilage.[2],[32] This was also observed in our study. Other studies have however shown that menisci contribute to joint space width and that meniscal protrusion may cause a reduction in radiographic tibiofemoral joint space width independent of cartilage thinning.[33],[34]
US detected femoral osteophytes in 13 knees which were not detected by CR while CR detected osteophytes in 12 knees that were not detected on US. The kappa value for agreement between US and CR was 74%. With CR as the gold standard, the sensitivity of US in demonstrating femoral osteophytes was 91.6% and the specificity was 82.2%. Similarly, US demonstrated tibial osteophytes in 10 knees that were not detected by CR while CR detected osteophytes in 9 knees that were not detected on US. The Kappa value for agreement between US and CR was 80% (P < 0.001). With CR as the gold standard, the sensitivity of US in detecting tibial osteophytes was 93.8% while the specificity was 85.9%. Similar findings were observed by Okano et al.[7] These findings show the significant role CR plays in the evaluation of OA. It is readily available and does not always require the radiologist to interpret for the managing physician.
The femoral condylar cartilage thickness in both knees in the OA group was significantly less (P < 0.001) compared to controls matched for age, sex, and BMI [Table - 4], showing that US measurement of knee femoral cartilage is useful in separating OA from asymptomatic knees. This finding agrees with a study carried out on patients with rheumatoid arthritis and OA.[35] They concluded that the thickness of knee cartilage in patients with rheumatoid arthritis was significantly less than that of their control group.
We experienced some limitations in our study. First, both US and CR evaluations were performed by the same researcher who ought to be blind to one of the investigations. The researcher however ensured that US was performed before assessing the plain radiographs. Second, there was no independent reference standard to confirm the abnormalities seen on sonography. None of the patients in the OA group had MRI or arthroscopy.
Conclusion
US provides a cheap, prompt and sensitive means of effectively evaluating knee OA. It is useful in assessing articular cartilage which plays an important role in the pathophysiology of OA. Synovial hypertrophy, effusion and Baker's cysts which are signs of inflammation are commonly seen and diagnosed on high-resolution US of the knee. This is particularly significant in our environment where other imaging modalities such as MRI are not readily available. Early detection and prompt management will thus improve patient's quality of life. US also has potential in monitoring the progression of OA. It is relevant even in late presentation. The findings we obtained in this study are similar to those previously reported.[25],[30],[31]
Ethical considerations
Approval for the study was obtained from the Ethical and Research Committee of the hospital.
Acknowledgments
We acknowledge our colleagues, Prof. VA Adetiloye, Dr. CM Asaleye, Dr. OO Ayoola and Dr. AD Omisore for their contributions to our work.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Authors contributions
We testify that we qualify for authorship. OCF conceived the study, SOO, BOI and OCF designed the study. SOO conducted the research under the supervision of BOI and OE. Statistical analysis was conducted mainly by ASA. Initial and final draft of the study were done by SOO and BOI. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Braun HJ, Gold GE. Diagnosis of osteoarthritis: Imaging. Bone 2012;51:278-88. [Google Scholar] |
| 2. | Wick MC, Kastlunger M, Weiss RJ. Clinical imaging assessments of knee osteoarthritis in the elderly: A mini-review. Gerontology 2014;60:386-94. [Google Scholar] |
| 3. | Favero M, Ramonda R, Goldring MB, Goldring SR, Punzi L. Early knee osteoarthritis. RMD Open 2015;1:e000062. [Google Scholar] |
| 4. | Sarmanova A, Hall M, Moses J, Doherty M, Zhang W. Synovial changes detected by ultrasound in people with knee osteoarthritis – A meta-analysis of observational studies. Osteoarthritis Cartilage 2016;24:1376-83. [Google Scholar] |
| 5. | Li Q, Amano K, Link TM, Ma CB. Advanced imaging in osteoarthritis. Sports Health 2016;8:418-28. [Google Scholar] |
| 6. | Shapiro LM, McWalter EJ, Son MS, Levenston M, Hargreaves BA, Gold GE, et al. Mechanisms of osteoarthritis in the knee: MR imaging appearance. J Magn Reson Imaging 2014;39:1346-56. [Google Scholar] |
| 7. | Okano T, Filippucci E, Di Carlo M, Draghessi A, Carotti M, Salaffi F, et al. Ultrasonographic evaluation of joint damage in knee osteoarthritis: Feature-specific comparisons with conventional radiography. Rheumatology (Oxford) 2016;55:2040-9. [Google Scholar] |
| 8. | Huang YP, Zhong J, Chen J, Yan CH, Zheng YP, Wen CY, et al. High-frequency ultrasound imaging of tidemarkin vitro in advanced knee osteoarthritis. Ultrasound Med Biol 2018;44:94-101. [Google Scholar] |
| 9. | Zhou XY, Zhang XX, Yu GY, Zhang ZC, Wang F, Yang YL, et al. Effects of low-intensity pulsed ultrasound on knee osteoarthritis: A meta-analysis of randomized clinical trials. Biomed Res Int 2018;2018:7469197. [Google Scholar] |
| 10. | Usenbo A, Kramer V, Young T, Musekiwa A. Prevalence of arthritis in Africa: A Systematic review and meta-analysis. PLoS One 2015;10:e0133858. [Google Scholar] |
| 11. | Akinpelu AO, Maduagwu SM, Odole AC, Alonge TO. Prevalence and pattern of knee osteoarthritis in a Northeastern Nigerian rural community. EAOJ 2011;5:5-12. [Google Scholar] |
| 12. | Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011;377:2115-26. [Google Scholar] |
| 13. | Loeuille D, Chary-Valckenaere I, Champigneulle J, Rat AC, Toussaint F, Pinzano-Watrin A, et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: Correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum 2005;52:3492-501. [Google Scholar] |
| 14. | Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol 1997;24:365-71. [Google Scholar] |
| 15. | Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: The MOST study. Ann Rheum Dis 2011;70:1804-9. [Google Scholar] |
| 16. | Atukorala I, Kwoh CK, Guermazi A, Roemer FW, Boudreau RM, Hannon MJ, et al. Synovitis in knee osteoarthritis: A precursor of disease? Ann Rheum Dis 2016;75:390-5. [Google Scholar] |
| 17. | Conaghan PG, D'Agostino MA, Le Bars M, Baron G, Schmidely N, Wakefield R, et al. Clinical and ultrasonographic predictors of joint replacement for knee osteoarthritis: Results from a large, 3-year, prospective EULAR study. Ann Rheum Dis 2010;69:644-7. [Google Scholar] |
| 18. | Möller I, Bong D, Naredo E, Filippucci E, Carrasco I, Moragues C, et al. Ultrasound in the study and monitoring of osteoarthritis. Osteoarthritis Cartilage 2008;16 Suppl 3:S4-7. [Google Scholar] |
| 19. | Naredo E, Cabero F, Palop MJ, Collado P, Cruz A, Crespo M, et al. Ultrasonographic findings in knee osteoarthritis: A comparative study with clinical and radiographic assessment. Osteoarthritis Cartilage 2005;13:568-74. [Google Scholar] |
| 20. | Koo S, Gold GE, Andriacchi TP. Considerations in measuring cartilage thickness using MRI: Factors influencing reproducibility and accuracy. Osteoarthritis Cartilage 2005;13:782-9. [Google Scholar] |
| 21. | Xu H, Bouta EM, Wood RW, Schwarz EM, Wang Y, Xing L, et al. Utilization of longitudinal ultrasound to quantify joint soft-tissue changes in a mouse model of posttraumatic osteoarthritis. Bone Res 2017;5:17012. [Google Scholar] |
| 22. | Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986;29:1039-49. [Google Scholar] |
| 23. | Friedman L, Finlay K, Jurriaans E. Ultrasound of the knee. Skeletal Radiol 2001;30:361-77. [Google Scholar] |
| 24. | Akinpelu AO, Alonge TO, Adekanla BA, Odole AC. Prevalence and pattern of symptomatic knee osteoarthritis in Nigeria: A community-based study. Internet J Allied Health Sci Pract 2009;7:1-7. [Google Scholar] |
| 25. | D'Agostino MA, Conaghan P, Le Bars M, Baron G, Grassi W, Martin-Mola E, et al. EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: Prevalence of inflammation in osteoarthritis. Ann Rheum Dis 2005;64:1703-9. [Google Scholar] |
| 26. | Gale DR, Chaisson CE, Totterman SM, Schwartz RK, Gale ME, Felson D, et al. Meniscal subluxation: Association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage 1999;7:526-32. [Google Scholar] |
| 27. | Aisen AM, McCune WJ, MacGuire A, Carson PL, Silver TM, Jafri SZ, et al. Sonographic evaluation of the cartilage of the knee. Radiology 1984;153:781-4. [Google Scholar] |
| 28. | Keen HI, Conaghan PG. Ultrasonography in osteoarthritis. Radiol Clin North Am 2009;47:581-94. [Google Scholar] |
| 29. | Felson DT, Niu J, Guermazi A, Sack B, Aliabadi P. Defining radiographic incidence and progression of knee osteoarthritis: Suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis 2011;70:1884-6. [Google Scholar] |
| 30. | Gaafar R, El-Ghobary MA, El-Gohary RM. The importance of using ultrasonography in knee osteoarthritis. Egypt J Intern Med 2012;24:93-6. [Google Scholar] |
| 31. | Wu PT, Shao CJ, Wu KC, Wu TT, Chern TC, Kuo LC, et al. Pain in patients with equal radiographic grades of osteoarthritis in both knees: The value of gray scale ultrasound. Osteoarthritis Cartilage 2012;20:1507-13. [Google Scholar] |
| 32. | Chatzopoulos D, Moralidis E, Markou P, Makris V, Arsos G. Baker's cysts in knees with chronic osteoarthritic pain: A clinical, ultrasonographic, radiographic and scintigraphic evaluation. Rheumatol Int 2008;29:141-6. [Google Scholar] |
| 33. | Breitenseher MJ, Trattnig S, Dobrocky I, Kukla C, Nehrer S, Steiner E, et al. MR imaging of meniscal subluxation in the knee. Acta Radiol 1997;38:876-9. [Google Scholar] |
| 34. | Miller TT, Staron RB, Feldman F, Cepel E. Meniscal position on routine MR imaging of the knee. Skeletal Radiol 1997;26:424-7. [Google Scholar] |
| 35. | Iagnocco A, Coari G, Zoppini A. Sonographic evaluation of femoral condylar cartilage in osteoarthritis and rheumatoid arthritis. Scand J Rheumatol 1992;21:201-3. [Google Scholar] |
Fulltext Views
4,248
PDF downloads
1,570





