Translate this page into:
Long-term outcome of giant cell tumors around the knee with associated pathological fractures treated by curettage and cementation
2 Department of Orthopaedic Surgery, Faculty of Medicine, Menoufia University, Menoufia, Egypt
3 Department of Orthopaedic Surgery, Al-Helal Hospital, Cairo, Egypt
4 Department of Orthopaedic Surgery, Faculty of Medicine, Menoufia University, Menoufia; Department of Orthopaedic Surgery, Keck School of Medicine, University of Southern California, Los Angeles, California, USA,
Corresponding Author:
Bahaa Z Hasan
Department of Orthopaedic Surgery, Faculty of Medicine, Menoufia University, Shebin El-Kom, Postal Code: 32511, Menoufia
Egypt
bahaa.hassan@med.menofia.edu.eg
| How to cite this article: Ebeid WA, Abo Senna WG, Mohamed MS, Hasan BZ, Kaissar BA, Badr IT, Mesregah MK. Long-term outcome of giant cell tumors around the knee with associated pathological fractures treated by curettage and cementation. J Musculoskelet Surg Res 2019;3:273-278 |
Abstract
Objectives: The management of giant cell tumor (GCT) lesions around the knee is challenging. This study aimed to investigate the long-term outcome of treating GCTs around the knee with associated pathological fractures by curettage and adjuvant cementation with the use of high-speed burr. Methods: Fifty patients with a mean age of 28.3 years were included in the study. The tumor was located in the distal femur (40 patients) and proximal tibia (10 patients). Eighteen patients were Grade II Campanacci, whereas 32 patients were Grade III. The mean follow-up period was 65 months. Twenty-nine patients (58%) underwent extended curettage and bone cement. Twenty-one patients (42%) underwent extended curettage, bone cement, and internal fixation. High-speed burr was used in 45 patients (90%). Bone graft was used in 8 patients (16%). Functional evaluation was done using the Musculoskeletal Tumor Society (MSTS) scoring system for the lower extremity. Results: The overall MSTS score was excellent in 42 patients (84%), good in 5 patients (10%), fair in 2 patients (4%), and poor in 1 patient (2%). The overall local recurrence rate was 12% (6 cases) and a 10% complication rate. There were no cases of fracture nonunion or distant metastasis. Conclusions: GCTs around the knee with associated pathological fractures at diagnosis can be treated with extended curettage using high-speed burr and adjuvant cementation with favorable functional long-term outcome.Introduction
Giant cell tumors (GCTs) represent 5% of primary bone tumors.[1],[2] GCTs are usually benign tumors, which have a locally aggressive behavior with occasional malignant transformation.[2],[3] With a slight female predominance, these tumors commonly occur in patients aged 20–40 years old. GCT of bone occurs most frequently in the distal femur, followed by the proximal tibia.[1],[2]
Most of the patients with GCTs present with progressive pain initially related to activity and later on become evident at rest. Pain is rarely severe unless the tumor becomes complicated by a pathological fracture, which is the first presentation in about 10%–30% of cases.[2],[3] Radiologically, the lesion is eccentrically located in the metaphysis of long bones usually about the subchondral bone. Moreover, it commonly breaks through the cortex.[3],[4]
Due to the benign nature of the tumor, the young age of patients, the predicted complications, and the need for revision intervention, the goal for joint preservation is generally reasonable.[5],[6],[7] The management of GCTs of bone by intralesional curettage with a filler such as polymethylmethacrylate (PMMA), autograft, allograft, or synthetic filler is the procedure of choice.[2],[3],[4] Cementation using PMMA is the most commonly utilized filler, and it has been studied in the literature with a good outcome.[3],[8] Cementation is efficient to allow instant stability and provide a sufficient quantity of filling material for tumor cavities.[8],[9] Moreover, its exothermic property acts as an adjuvant and decreases the recurrence rate.[8],[9],[10] However, its use in the management of pathological fractures has not been notably studied.
Curettage and cementation option for pathological fractures was firstly reported by Wouters [11] in 1974 and Persson et al.[12] in 1984. The recurrence rate after curettage or resection differs significantly in the literature, and it is unclear if curettage is ideal after a pathological fracture.[5] Moreover, it is not clear in the literature if extended curettage and cement alone without internal fixation is enough, regarding the functional outcome, fracture union, complications, and recurrence rate.[13],[14]
This study was done retrospectively to evaluate the long-term functional and oncological outcomes of GCTs around the knee with associated pathological fractures treated with curettage and adjuvant cementation and to assess the effect of extended curettage using high-speed burr on the outcome.
Materials and Methods
After taking approval from our ethical committee, we retrospectively reviewed 50 patients with GCTs around the knee joint (distal femur or proximal tibia), who developed pathological fractures at diagnosis or before surgical intervention, treated by curettage and cement augmentation between 2000 and 2015, at a single orthopedic institute. The available clinical and radiological records of all patients were analyzed and reviewed.
Data collection included demographic data, radiological evaluation, histopathological diagnosis, tumor site and extent, Campanacci grading system,[15] fracture characteristics, fracture healing, type of surgical intervention, complications, follow-up, and functional evaluation [Figure - 1].
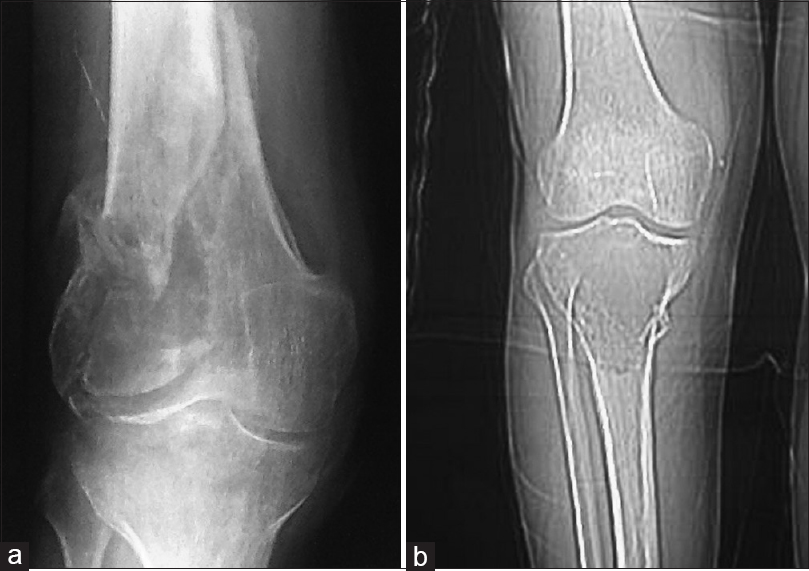 |
| Figure 1: Anteroposterior radiographs showing a giant cell tumor with a pathological fracture in (a) distal femur. (b) Proximal tibia |
All patients with pathological fractures following GCTs around the knee managed with curettage and cementation, with or without internal fixation, and a follow-up period of at least 3 years were included in this study. Cases were confirmed histopathologically to be GCT. Patients with malignant GCTs and those who received adjuvant radiotherapy and/or chemotherapy were excluded. Patients who received denosumab alone or as adjuvant before surgery were excluded, as this study was held over a long period of time and the introduction of denosumab was late.
Operative technique
Preoperative confirmation of diagnosis was done using computed tomography (CT)-guided core biopsy and less frequently, by direct open biopsy. Curettage and cement augmentation were done for all patients without waiting for the fracture to heal. Patients received general or regional anesthesia and positioned supine. A tourniquet was applied with no exsanguination. The incision was planned respecting tumor resection rules allowing adequate exposure of the whole lesions and large adequate windows either through the weakest point or through the fracture site. Adequate thorough curettage was done using variable-sized curettes and copious irrigation with hydrogen peroxide and jet saline until removal of any suspicious macroscopic tissue was assured. Curettage was extended with the use of high-speed burr in 45 cases (90%) to clean up the resultant cavity going tangentially in areas with the exposed subchondral bone [Figure - 2]. In the remaining 5 cases, the high-speed burr was not available; this was especially in the early cases of this study. Iliac crest bone graft was used in 8 patients (16%). It was placed over the subchondral bone before packing the rest of the cavity with cement, to avoid the articular damage due to the thermal energy generated by the cement. Moreover, if any recurrence occurred, removal of the cement would not damage the articular surface. A small quantity of cement was applied, in the other cases, near the adjacent articular surface to form a thin cement layer. Then, we waited until it consolidated, and then, the remaining cavity was filled [Figure - 3].
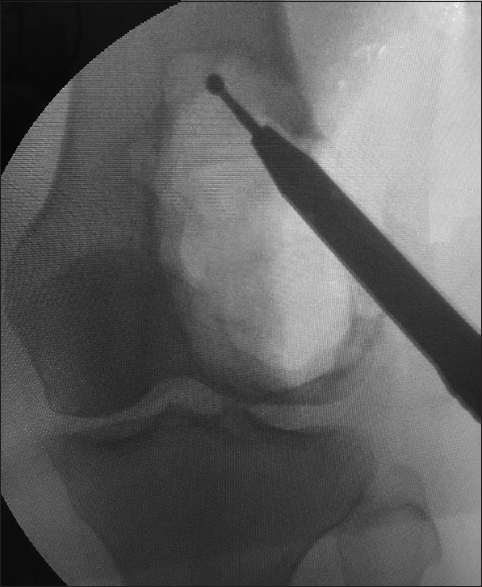 |
| Figure 2: Intraoperative radiograph of the distal femur giant cell tumor cavity while using a high-speed burr |
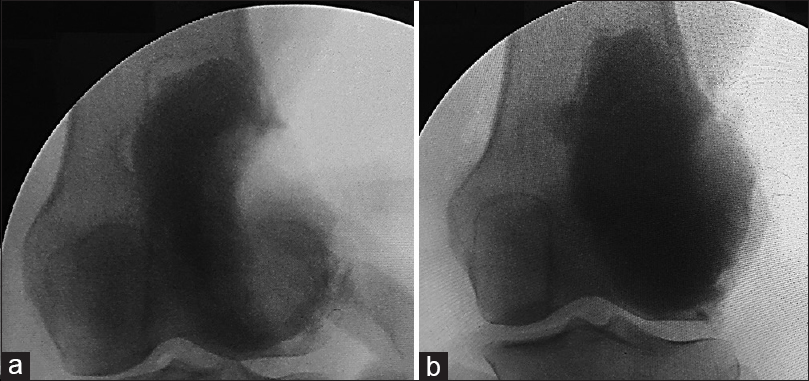 |
| Figure 3: Intraoperative image intensifier photos. (a) While applying the first layer of cement. (b) After filling the cavity with cement |
The type of fracture determined the mode of treatment. Twenty-nine patients (58%) presented with extra-articular nondisplaced fractures and underwent extended curettage and bone cement only [Figure - 4]. Fixation of the fracture with plates and screws was done in 14 patients (28%) who presented with displaced extra-articular or intra-articular fractures that required anatomical reduction to achieve more stability [Figure - 5]a. In 7 patients (14%) with large cavities and extra-articular minimally displaced fractures, intramedullary Steinmann pins were placed for reinforcement of the fractured bone and to allow an earlier range of motion [Figure - 5]b.
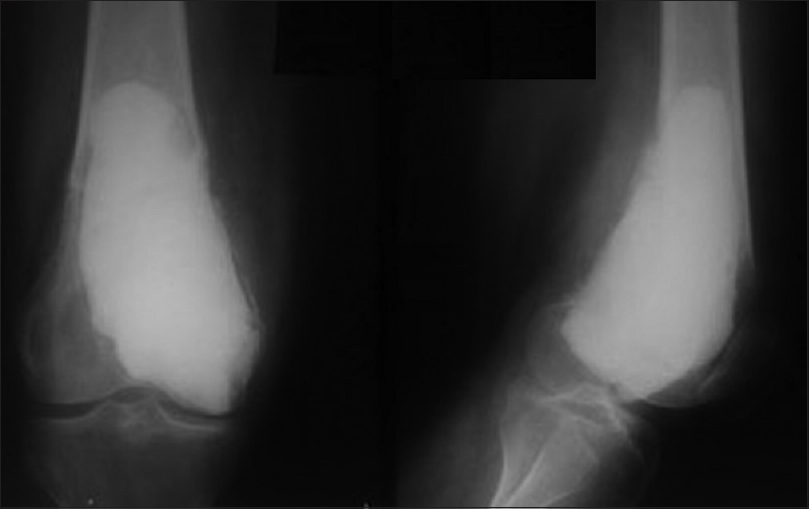 |
| Figure 4: Postoperative anteroposterior and lateral radiographs after curettage and cement augmentation of a giant cell tumor complicated by supracondylar femoral fracture |
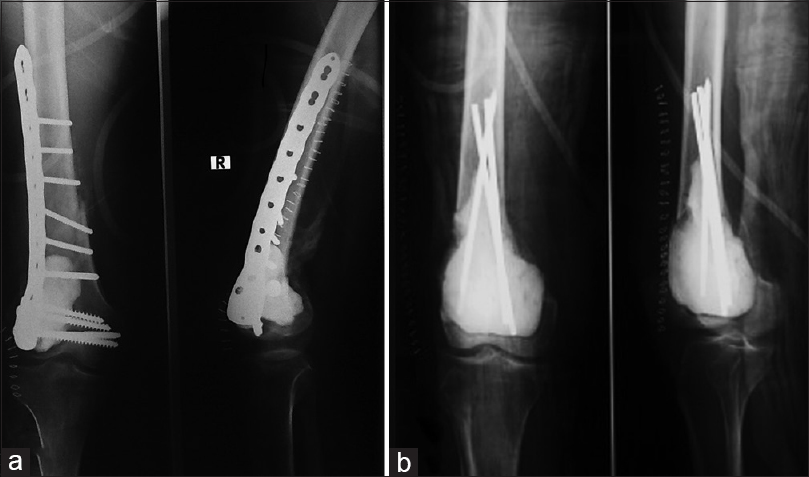 |
| Figure 5: Postoperative anteroposterior and lateral radiographs showing internal fixation after curettage and cementation of giant cell tumor with a pathological distal femoral fracture. (a) Internal fixation by distal femoral locked plate. (b) Augmentation with three Steinmann pins |
Plain radiographs were taken in the first postoperative day, and appropriate antibiotics were administrated. Wound condition was observed during the first 2 weeks postoperatively. Active, active-assisted, and passive range of motion exercises (seated and supine), isometric quadriceps, hamstring, and gluteal muscle exercises, straight-leg raising exercises, and strengthening exercises were done depending on the stability of the fracture in each case and as tolerated by the patient. Partial weight-bearing was started in the first 6–12 weeks. With fracture consolidation, full weight-bearing was started.
All patients had a clinical evaluation at 2, 6, and 12 weeks postoperatively, then every 3 months till the end of the first 2 years, then every 6 months in the third year, then once yearly in the following years [Figure - 6]. Radiological evaluation by plain radiographs of the knee was done in every scheduled visit after surgery to evaluate fracture union and to detect any signs of recurrence. If any recurrence was suspected, magnetic resonance imaging and/or CT was requested. Moreover, plain radiographs of the chest were done to detect any distant metastasis. The Musculoskeletal Tumor Society (MSTS) score for lower extremity [16] was used for assessment of the functional outcome.
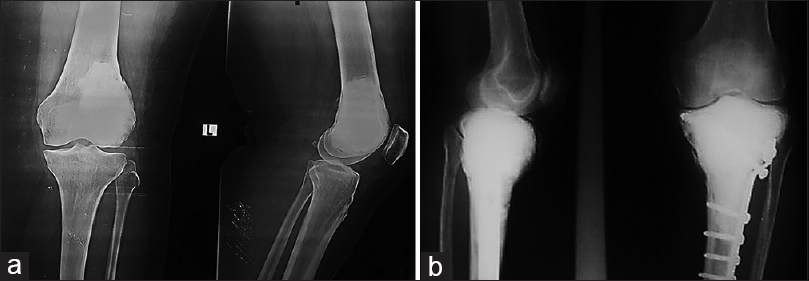 |
| Figure 6: (a) Follow-up radiographs at 18 months a case of giant cell tumor at the distal end of the femur with a pathological fracture managed by curettage and cementation. (b) Follow-up radiographs at 26 months a case of giant cell tumor at the proximal end of the tibia with a pathological fracture managed by curettage, cementation, and plate fixation |
Statistical analysis
IBM SPSS software version 25 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis of data, focusing on epidemiological characteristics, functional and oncological outcomes, complications, and recurrence. Continuous variables as age and follow-up period were expressed as mean and range. Complications and recurrence were described as a percentage. The independent-samples t-test and one-way ANOVA test were used to compare the means between the groups. Chi-square test was used to study the association between each group and the recurrence.
Results
This study included 50 patients – 22 males (44%) and 28 females (56%). The mean age was 28.3 years (range, 16–53). Seven patients (14%) presented initially with recurrent GCT, whereas the rest of the patients (86%) were de novo lesions. The tumor was in the distal femur in 40 patients (80%) and proximal tibia in 10 patients (20%). Eighteen patients (36%) were Grade II Campanacci, whereas 32 patients (64%) were Grade III. The mean follow-up was 65 months (range, 37–120) [Table - 1].

Patients were classified into two groups according to the diagnosis. Group 1 was 43 patients (86%) primarily diagnosed as GCT at our institute. Group 2 was 7 patients (14%) presented as a recurrent GCT. Patients also were classified into three groups according to the type of surgical intervention. Group A, with extra-articular undisplaced fractures, included 29 patients (58%) who underwent extended curettage and bone cement. Group B, with extra-articular displaced or intra-articular fractures, included 14 patients (28%) who underwent extended curettage, bone cement, and internal fixation by plates and screws. Group C, with minimally displaced extra-articular fractures, included 7 patients (14%) who underwent extended curettage, bone cement, and internal fixation with pins.
Fracture healing occurred in all cases after a mean of 13 weeks (range, 7–36), with no statistically significant difference between Group 1 and Group 2 (P = 0.56) or between Group A, Group B, and Group C (P = 0.79). The overall functional outcome according to the mean MSTS score at the final follow-up time was 27.8 (range, 18–30), with excellent score in 42 patients (84%), good in 5 patients (10%), fair in 2 patients (4%), and poor in 1 patient (2%).
At the final follow-up, the functional outcome according to the MSTS scoring system was found to be significantly higher in Group 1 than Group 2 (P = 0.020). As regards the relationship between functional outcome and type of procedure, the functional outcome was significantly higher in Group A than Group B (P = 0.026) [Table - 2].

The mean operative time was 1.9 h (range, 1–3). There was an inverse correlation between operative time and functional outcome with a statistically significant difference (P = 0.023).
The overall local recurrence rate was 12% (6 patients). Five of them were treated with joint preservation, whereas one patient presented in a late stage with joint destruction and was treated with wide excision and endoprosthesis. There was no statistically significant difference between Group 1 and Group 2 and between Group A, Group B, and Group C regarding the local recurrence rate. Using high-speed burr during the procedure was statistically significant (P = 0.042) [Table - 3].

Regarding complications other than local recurrence, we had an overall complication rate of 10%. Early complications included one patient with deep-seated infection with a sinus (2%). Late complications were one patient with varus internal rotation deformity in the tibia and one patient with knee flexion deformity of 20°. Moreover, one patient developed chronic anterior knee pain due to prominent hardware that was removed later on. One diabetic patient suffered from superficial thrombophlebitis. We had no cases of fracture nonunion or distant metastasis.
Discussion
In this study, the overall functional outcome according to the MSTS score was excellent in 42 patients (84%), good in 5 patients (10%), fair in 2 patients (4%), and poor in 1 patient (2%). Gupta and Garg [17] stated that GCTs with pathological fracture can be treated with thorough curettage and cementation with an excellent outcome. Using the MSTS scoring, the results were excellent (72%), good (12.82%), fair (10.25%), and poor (5.12%).
O'Donnell et al.[18] concluded that the existence of pathologic fractures would be accompanied with a higher rate of recurrence after curettage owing to contamination of nearby soft tissues. In the current study, the local recurrence rate was 12% in a mean follow-up period of 65 months. When a high-speed burr was used, the local recurrence was 8%. The use of hydrogen peroxide as an adjuvant in all patients may further have contributed to reducing the chance of local recurrence. In similar studies in the literature, Hu et al.[6] had a recurrence rate of 28%, and Gupta and Garg [17] had a recurrence of 10.5%. The recurrence rate in Dreinhöfer et al. study [19] was 26.66% and in van der Heijden et al. study [5] was 30%. On the other hand, the incidence of local recurrence was lower in studies where en bloc marginal resection and arthroplasty were done. Hu et al.[6] had a recurrence rate of only 4.9%, and van der Heijden et al.[5] had 0% recurrence for the resection group.
Deheshi et al.[7] compared a group of GCT patients who presented with pathological fractures and another group of GCT patients without pathological fractures; both treated with joint preservation surgery. They reported a recurrence rate of 17.4% in the fractured group and 22.1% in the nonfractured group.[7]
Concerning whether pathologic fracture treated by joint preservation surgery can lead to inferior oncologic results, our study proved that the oncological outcome of these patients was the same or even sometimes better than joint replacement.
Some authors compared the outcome of resection versus joint preservation in GCT with a pathological fracture. van der Heijden et al.[5] reported higher MSTS scores after curettage with adjuvants, mean 28 (range, 23–30), compared with resection, mean 25 (range, 13–30).
In Natarajan et al. study,[20] the functional results were evaluated using Enneking criteria. Excellent results were achieved in 90 patients (62%), and 39 patients (27%) had good results.
Hu et al.[6] found that the median (range) MSTS score was 26.0 (0–30) in patients treated with en bloc marginal resection. This was considered less than functional outcomes in our study. Li et al.[21] also found that the mean functional score according to the MSTS score was 81% in patients who received endoprosthesis replacement and 82% in other reconstruction options.
In this study, we had a complication rate of 10% in the form of chronic infection with a sinus, varus internal rotation deformity in the tibia, knee flexion deformity of 20 degrees, anterior knee pain, and superficial thrombophlebitis. None of our patients developed fracture nonunion or metastasis. These complications were much lower than those reported by studies in which en bloc resection and endoprosthesis were used. Natarajan et al.[20] complications were periprosthetic fracture (8.3%), infection (6.9%), and aseptic loosening (4.2%). Moreover, recurrence was reported in one case (0.69%) and was treated with wide local excision. Overall, from 143 patients, prosthesis removal was done in 11 cases, and amputation was done in 3 cases. In van der Heijden et al. study,[5] the complication rate after curettage with adjuvants was 4% (1/23) and 16% after en bloc resection (4/25).
In this study, we found that the age, sex, and the tumor location either distal femur or proximal tibia had no statistically significant affection on the functional and oncological outcomes. In our study, the functional outcome was better in patients who had extended curettage and cement alone. The fact that the cement did not reduce the functional outcome in this study was consistent with the same results reported by Kafchitsas et al.[22] and von Steyern et al.[23]
The use of high-speed burr showed a significant difference in the recurrence rate, which is consistent with what is published in the literature.[17],[19],[24] In this study, when high-speed burr was not used, recurrence occurred in 2 cases out of 5 cases (40%). When high-speed burr was used, recurrence occurred in only 4 cases out of 45 cases (8.9%).
None of the cases in the current study experienced degenerative changes in the knee joint with long-term follow-up which indicates that the fear of subsequent osteoarthritis is largely untruthful. Our results suggest that subchondral cement does not cause degenerative arthritis provided that the articular cartilage continuity is maintained under surgery.
Recurrence rate should always be in mind when treating GCT.[25] Recurrence is lower after resection and reconstruction compared to curettage.[20] We believe that whenever possible and indicated, curettage with local adjuvants should be done. If joint preservation is possible, GCT presented with a pathologic fracture can be curetted and cemented. This avoids the higher complication rate with the prosthesis with the future need for revision surgery and sometimes decreased function. Joint preservation is a good option for GCT which is a benign tumor occurring in relatively young patients. Resection and endoprosthesis should be reserved for complicated intra-articular fractures, fractures with large soft tissue extension, or if structural integrity is difficult to be regained. Furthermore, curettage and cementation can be repeated for recurrence when it is difficult for endoprosthetic reconstruction. Our current choices for endoprosthesis are multiple recurrent GCT, impossible joint salvage, and extensive soft-tissue involvement.
This study has some limitations being a retrospective study with selection bias of groups. Although the 50 patients included in this study are considered a relatively high number treated by a single orthopedic institute, still the number of patients was small due to the low prevalence of GCT. As we only included patients with GCT associated with pathological fractures, the outcome and recurrence rates following treatment of GCT patients without pathological fractures cannot be deduced from this study and need to be addressed in future studies.
Conclusions
Extended curettage with the use of high-speed burr and filling the cavity with PMMA cement is considered a good option for treatment of GCT with associated pathological fracture. It has a predictable satisfactory functional outcome provided that anatomic reduction and stable fixation can be achieved.
Ethical consideration
This study was approved by the ethical committee of our institution and every participant signed an informed consent.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Authors' contributions
WAE, WGA and MSM conceived and designed the study, conducted research, provided and organized data. WGA, MSM and BZH collected, analyzed, and interpreted data. WAE and BZH provided research material and logistic support. BAK, ITB and MKM reviewed the literature and wrote initial and final drafts of the article. MKM contributed with clinical studies, data acquisition, data analysis, statistical analysis, manuscript preparation, and manuscript editing. All authors have critically revised and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Kim Y, Nizami S, Goto H, Lee FY. Modern interpretation of giant cell tumor of bone: Predominantly osteoclastogenic stromal tumor. Clin Orthop Surg 2012;4:107-16. [Google Scholar] |
| 2. | Sobti A, Agrawal P, Agarwala S, Agarwal M. Giant cell tumor of bone – An overview. Arch Bone Jt Surg 2016;4:2-9. [Google Scholar] |
| 3. | Amanatullah DF, Clark TR, Lopez MJ, Borys D, Tamurian RM. Giant cell tumor of bone. Orthopedics 2014;37:112-20. [Google Scholar] |
| 4. | van der Heijden L, Dijkstra PD, van de Sande MA, Kroep JR, Nout RA, van Rijswijk CS, et al. The clinical approach toward giant cell tumor of bone. Oncologist 2014;19:550-61. [Google Scholar] |
| 5. | van der Heijden L, Dijkstra PD, Campanacci DA, Gibbons CL, van de Sande MA. Giant cell tumor with pathologic fracture: Should we curette or resect? Clin Orthop Relat Res 2013;471:820-9. [Google Scholar] |
| 6. | Hu P, Zhao L, Zhang H, Yu X, Wang Z, Ye Z, et al. Recurrence rates and risk factors for primary giant cell tumors around the knee: A multicentre retrospective study in China. Sci Rep 2016;6:36332. [Google Scholar] |
| 7. | Deheshi BM, Jaffer SN, Griffin AM, Ferguson PC, Bell RS, Wunder JS. Joint salvage for pathologic fracture of giant cell tumor of the lower extremity. Clin Orthop Relat Res 2007;459:96-104. [Google Scholar] |
| 8. | Bini SA, Gill K, Johnston JO. Giant cell tumor of bone. Curettage and cement reconstruction. Clin Orthop Relat Res 1995;321:245-50. [Google Scholar] |
| 9. | Alkalay D, Kollender Y, Mozes M, Meller I. Giant cell tumors with intraarticular fracture. Two-stage local excision, cryosurgery and cementation in 5 patients with distal femoral tumor followed for 2-4 years. Acta Orthop Scand 1996;67:291-4. [Google Scholar] |
| 10. | Puri A, Agarwal M. Treatment of giant cell tumor of bone: Current concepts. Indian J Orthop 2007;41:101-8. [Google Scholar] |
| 11. | Wouters HW. Giant cell tumor of the distal end of the femur with intra-articular fracture of the knee. Treatment by excochleation and filling with bone cement. Rev Chir Orthop Reparatrice Appar Mot 1974;60 Suppl 2:316. [Google Scholar] |
| 12. | Persson BM, Ekelund L, Lövdahl R, Gunterberg B. Favourable results of acrylic cementation for giant cell tumors. Acta Orthop Scand 1984;55:209-14. [Google Scholar] |
| 13. | Zheng K, Yu XC, Hu YC, Wang Z, Wu SJ, Ye ZM. How to fill the cavity after curettage of giant cell tumors around the knee? A multicenter analysis. Chin Med J (Engl) 2017;130:2541-6. [Google Scholar] |
| 14. | Gitelis S, Mallin BA, Piasecki P, Turner F. Intralesional excision compared with en bloc resection for giant-cell tumors of bone. J Bone Joint Surg Am 1993;75:1648-55. [Google Scholar] |
| 15. | Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am 1987;69:106-14. [Google Scholar] |
| 16. | Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 1993;286:241-6. [Google Scholar] |
| 17. | Gupta SP, Garg G. Curettage with cement augmentation of large bone defects in giant cell tumors with pathological fractures in lower-extremity long bones. J Orthop Traumatol 2016;17:239-47. [Google Scholar] |
| 18. | O'Donnell RJ, Springfield DS, Motwani HK, Ready JE, Gebhardt MC, Mankin HJ. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am 1994;76:1827-33. [Google Scholar] |
| 19. | Dreinhöfer KE, Rydholm A, Bauer HC, Kreicbergs A. Giant-cell tumours with fracture at diagnosis. Curettage and acrylic cementing in ten cases. J Bone Joint Surg Br 1995;77:189-93. [Google Scholar] |
| 20. | Natarajan MV, Prabhakar R, Mohamed SM, Shashidhar R. Management of juxta articular giant cell tumors around the knee by custom mega prosthetic arthroplasty. Indian J Orthop 2007;41:134-8. [Google Scholar] |
| 21. | Li X, Guo W, Yang Y, Wei R, DU ZY. Surgical treatment for long bone giant cell tumor of extremity with pathologic fracture. Beijing Da Xue Xue Bao Yi Xue Ban 2013;45:745-51. [Google Scholar] |
| 22. | Kafchitsas K, Habermann B, Proschek D, Kurth A, Eberhardt C. Functional results after giant cell tumor operation near knee joint and the cement radiolucent zone as indicator of recurrence. Anticancer Res 2010;30:3795-9. [Google Scholar] |
| 23. | von Steyern FV, Kristiansson I, Jonsson K, Mannfolk P, Heinegård D, Rydholm A. Giant-cell tumour of the knee: The condition of the cartilage after treatment by curettage and cementing. J Bone Joint Surg Br 2007;89:361-5. [Google Scholar] |
| 24. | Errani C, Ruggieri P, Asenzio MA, Toscano A, Colangeli S, Rimondi E, et al. Giant cell tumor of the extremity: A review of 349 cases from a single institution. Cancer Treat Rev 2010;36:1-7. [Google Scholar] |
| 25. | Salunke AA, Chen Y, Chen X, Tan JH, Singh G, Tai BC, et al. Does pathological fracture affect the rate of local recurrence in patients with a giant cell tumour of bone? A meta-analysis. Bone Joint J 2015;97-B: 1566-71. [Google Scholar] |
Fulltext Views
1,885
PDF downloads
447





