Translate this page into:
Nerve transfers following cervical spinal cord injury: A review and reconstructive algorithm
2 Birmingham Hand Centre, New Queen Elizabeth Hospital Birmingham, Edgbaston, Birmingham, UK
Corresponding Author:
Joseph A Ward
Department of Plastic Surgery, Royal Marsden NHS Foundation Trust, 203 Fulham Road, Chelsea, SW3 6JJ London
UK
joseph.a.ward@btinternet.com
| How to cite this article: Ward JA, Power DM. Nerve transfers following cervical spinal cord injury: A review and reconstructive algorithm. J Musculoskelet Surg Res 2019;3:152-160 |
Abstract
Background: Cervical spinal cord injury (CSCI) is a devastating consequence of trauma. Restoration of upper limb function can improve quality of life, reduce long-term care needs and is highly rated by patients. Methods: We performed a non-systematic review of all studies reporting nerve transfer in CSCI to derive a putative reconstructive algorithm based primarily on nerve transfers. Results: For CSCIs above C5, no intraplexal donors exist. For CSCIs at C5 or below, axillary nerve (C5) branches may be transferred to triceps to restore elbow extension, musculocutaneous nerve (C6) branches may be transferred to the median nerve to restore pronation/ finger flexion whilst nerve branches to supinator (C6) may be transferred to re-innervate finger extensors. Further functional gains such as re-innervation of hand intrinsics, accessory respiratory function and postural control of the trunk may be possible but are not reported. Conclusions: Nerve transfers following CSCI represent an emerging area of upper limb surgery where bespoke surgical strategies undertaken early during rehabilitation course have the potential to change functional outcomes.
“To someone who has nothing, a little is a lot.”
– Sterling Bunnell
Introduction
Cervical spinal cord injury (CSCI) is a devastating consequence of trauma. In the US, it has an incidence of 24 cases per million population, and over 166,000 people are thought to live with tetraplegia.[1] Patients are often young, healthy, and economically active individuals that lose their independence to significant personal and societal cost.[2],[3] One of the most disabling sequelae of CSCI is the loss of upper limb function.[4] Multiple surveys of tetraplegic patients demonstrate that patients rate return of upper limb function very highly.[5] This is unsurprising when considered that improved function can facilitate greater ability of patients to self-feed, self-catheterize, wash, and partially assist carers with transfers. In one survey of 681 spinal cord injury patients, tetraplegic respondents ranked return of hand function above control of bladder, bowel, and sexual functions as well as chronic pain.[6] In another survey of tetraplegic patients, 77% of respondents felt that improved hand function would lead to an important or very important improvement in quality of life.[7]
The return of upper limb function following CSCI can be achieved through the use of tendon transfers, arthrodesis, or nerve transfer procedures. Of these procedures, nerve transfers demonstrate early promise with selective nerve fascicle transfer providing scope for restoration of prehensile grip with minimal surgical morbidity and limited sacrifice of donor muscle innervation.[8] Nerve transfers for restoration of upper limb function are not new and have been widely undertaken following brachial plexus and peripheral nerve injuries. However, more recently, there has been increased interest following CSCI. In this paper, we summarize the published nerve transfer work for CSCI to date supplementing previous work[9],[10] in a nonsystematic literature review, we also formulate a hypothetical and putative algorithm to guide surgical reconstruction of upper limb function based primarily on nerve transfers that may be used to inform surgical decision-making.
Materials and Methods
A non-systematic literature review of all identifiable published works available through PubMed reporting nerve transfer following traumatic CSCI was undertaken. The following search terms were employed in combination or alone: “nerve transfer,” “tetraplegia,” “quadriplegia,” and “cervical spinal cord injury.” The reference list of every identifiable article was examined for further relevant studies. Studies not written or translated into English were excluded. No studies were excluded on the basis of publication date. Every study identified through the literature review was reviewed by the primary author, and in discussion with the senior author, an algorithm for reconstructing upper limb function based primarily on nerve transfer was synthesized.
Results
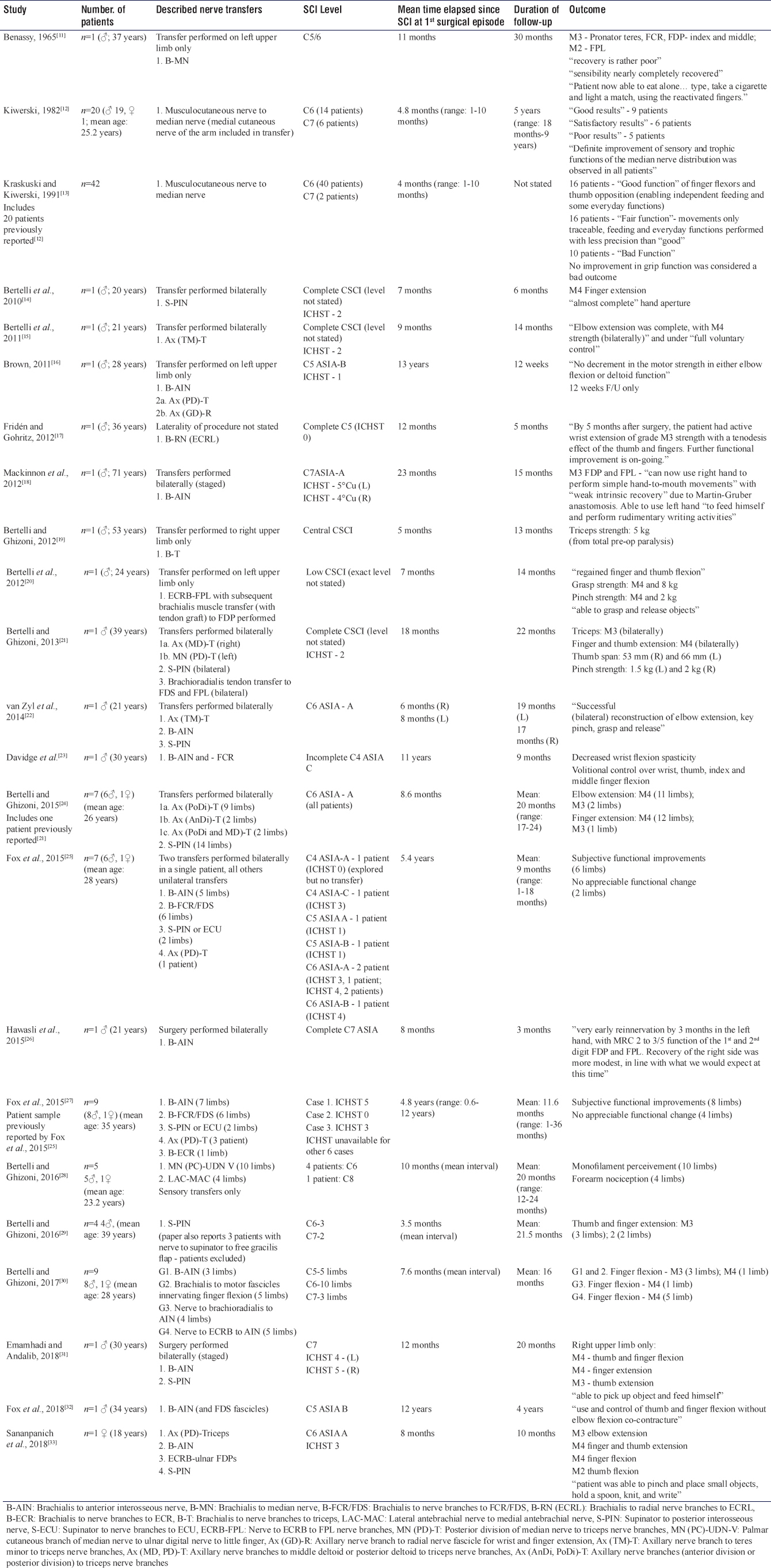
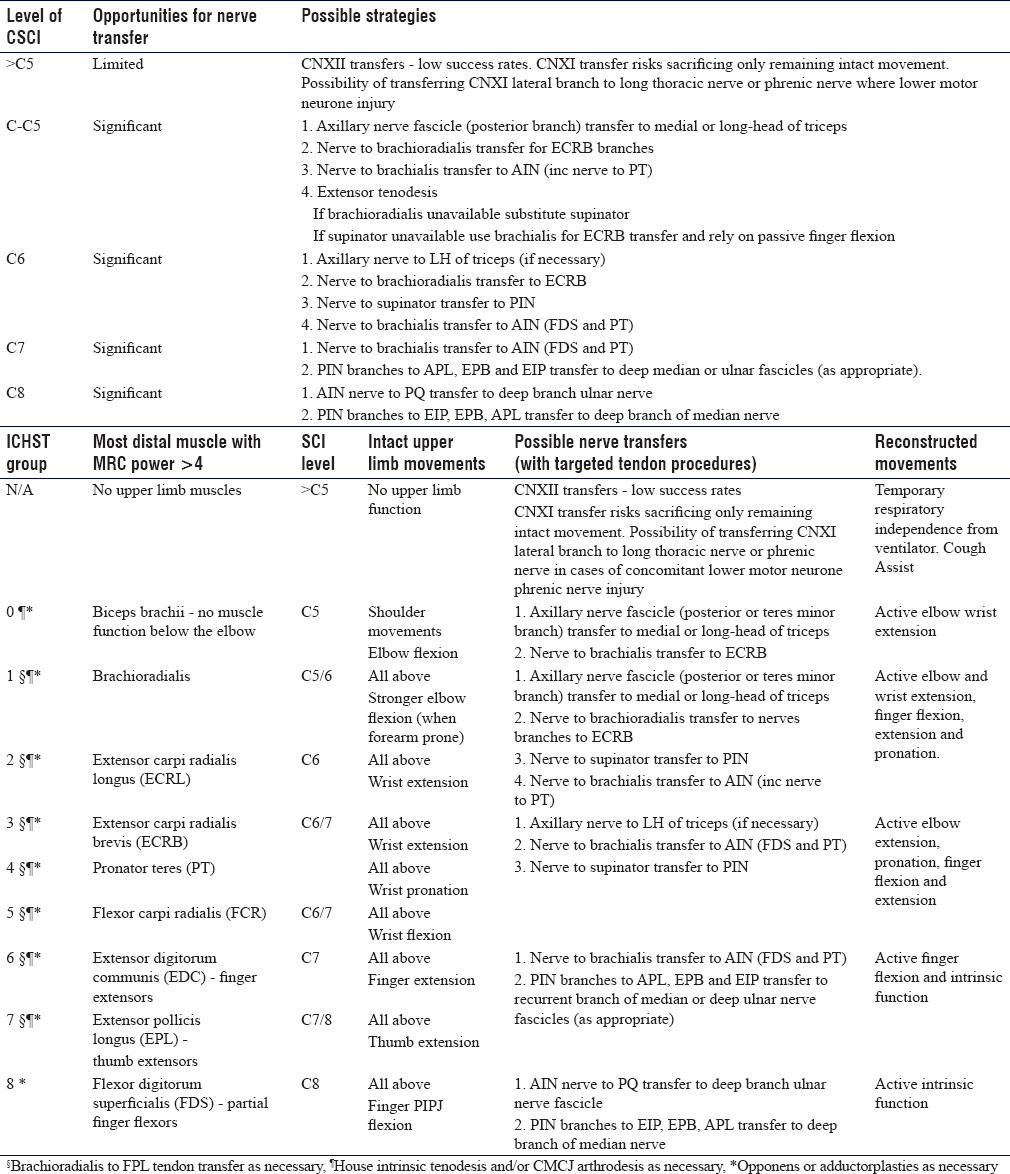
We identified 23 published studies that reported nerve transfer in 101 patients since 1965 and these are summarized in [Table - 1]. The mean age of patients that received nerve transfer was 28.1 years (range: 12–72). The median number of nerve transfers performed per patient was 1 (range: 1–4). The mean time that had elapsed between CSCI and first episode of surgery per study was 28.1 months (range: 4–156) while the mean duration of follow-up per study was 16.6 months (range: 3–60). After close appraisal of each study identified, a reconstructive algorithm was constructed and stratified according to CSCI level. The reconstructive algorithm is outlined in [Table - 2].
Discussion
In constructing our algorithm, we prioritized functional gains into a hierarchy of movements [Figure - 1]: active elbow extension, active wrist extension, active finger flexion, active finger extension, and active intrinsic muscle function targeting maximal functional gain for least opportunity cost. Where possible, we have been mindful of synergistic movements through the tenodesis effect, for instance, targeting active wrist extension to harness passive finger flexion. We have chosen to prioritize proximal muscle reinnervation, in particular, triceps function because this facilitates the positioning of the upper limb in space and also allows the elbow to act as a stable post for distal musculature.
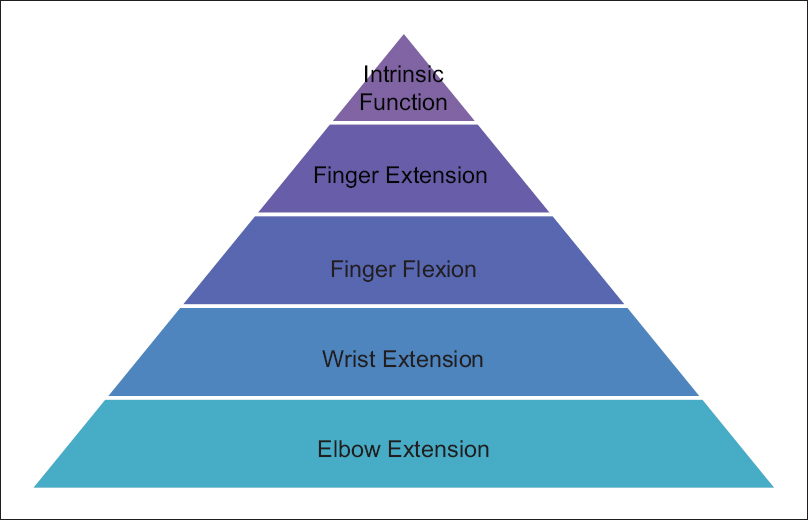 |
| Figure 1: Hierarchy of prioritized movements |
We identified a paucity of possible nerve transfers for CSCIs above C5 due to the limited donors but increasingly significant opportunities for reconstruction of upper limb function between C5 and C8. We have chosen to base the algorithm on several key transfers, in particular, the transfer of a fascicle from the axillary nerve to the long head of triceps through an anterior approach, nerve to brachialis transfer to the anterior interosseous nerve (AIN), and nerve branches to supinator transfer to the distal posterior interosseous nerve (PIN). These are transfers that have been employed by a number of surgeons in the literature with increasing international experience. We avoided extraplexal transfers to ensure donor morbidity was limited to the upper limb.
CSCIs above C5
For CSCIs above C5, only muscles innervated by the cervical plexus, phrenic nerve, or cranial nerves retain innervation limiting surgical options. Cranial nerve transfers (accessory and hypoglossal nerve) have been described in obstetric[34],[35] and adult traumatic[36] brachial plexus injuries with good success but high donor morbidity: sacrifice of ipsilateral volitional tongue and neck function. In CSCI patients who already have very little volitional control over any movement at the outset, 50% loss of tongue movement devastatingly impedes chewing and swallowing and could prevent control of a wheelchair using tongue or mouth controls. Similarly, accessory nerve transfer while technically feasible[37],[38] carries significant morbidity: ipsilaterally reducing retained neck movement and accessory muscles of respiration (in patients with already compromised respiratory function) and weakening retained scapular stability. In this context, We do not agree that extraplexal nerve transfers to the upper limb should be undertaken. In cases of high CSCI where there is concomitant lower motor neuron phrenic nerve dysfunction due to extensive C3–5 nerve root injury, a targeted transfer of the spinal accessory nerve may provide cough assist and achieve temporary independence from ventilation. This transfer has been demonstrated to be technically feasible in cadaveric studies,[39],[40] but no clinical case reports of its use have been published to date.
C5–6 injuries
For mid-cervical CSCIs (C5–6), a combination of three to four nerve transfers are possible and these can be supplemented by tendon transfers where necessary. For restoration of elbow extension, the transfer of axillary nerve (posterior branch) onto the medial or long head of triceps is the best option. Published work has employed the anterior or posterior divisions of the axillary nerve with equivalent efficacies, and anatomically, the divisions are equivalent in diameter and number of myelinated nerve fibers and demonstrate little variation.[41] Interestingly, it has been shown that the risk of deltoid denervation is low due to its dual innervation from both the anterior and posterior branches of the axillary nerve in 89.1% of patients.[42] We suggest the transfer of the posterior division due to its greater proximity to the radial nerve triceps branches and the redundant nature of its original innervation (shoulder extension) in tetraplegia, but difficulties in isolated clinical testing of teres minor motor function preoperatively may result in clinical uncertainty.
For restoration of wrist extension, we consider transfer of nerve branch to supinator or brachioradialis to extensor carpi radialis brevis (ECRB) to be the best option. This is based on the rationale that provision of wrist extension facilitates passive finger flexion and extension through the tenodesis effect. Where an intact extensor carpi radialis longus exists, restoration of ECRB function augments the strength of wrist extension and tames radial deviation. When brachioradialis innervation is unavailable for provision of wrist extension, nerve to supinator should be employed, however, where this is unavailable nerve to brachialis may be used with subsequent acceptance of passive finger flexion. Nerve to supinator or brachioradialis transfer to ECRB has not been clinically described, but the former has been shown to be anatomically feasible[43] with overlap between ECRB and supinator nerve branches arising from the PIN and a mean of 2.3 nerve branches to the supinator muscle providing redundancy.[44]
For provision of active finger flexion, nerve to brachialis transfer to AIN should be employed (flexor digitorum superficialis [FDS] and pronator teres [PT] branches). This is commonly reported in the literature and carries low donor morbidity where biceps brachii function remains intact. The transfer also provides the possibility of restoring thumb function, although brachioradialis to flexor pollicis longus tendon transfer may provide a stronger key pinch.[21] Theoretically reinnervation of flexor carpi ulnaris is possible although the functional gain would be limited when wrist extension is intact because wrist flexion can be brought about through gravity. The brachialis to AIN transfer necessitates the dissection of FDS and PT fascicular bundles from the AIN making the procedure technically more involved than other transfers. Nonetheless, its near ubiquitous use in the literature underpins potential for restoring finger flexion where other transfers are unavailable. For patients with C5–6 CSCIs where nerve to supinator is unavailable for PIN transfer, finger extensor tenodesis is recommended for finger extension due to the paucity of other nerve transfer strategies available for restoring active finger extension.
C6/7 injuries
For injuries at this level, the recommended algorithm is similar to that for injuries at C5–6 and C6 although reasonable wrist extension can be assumed. Good wrist extension allows guaranteed availability of nerve branch to supinator transfer to PIN for restoration of active finger extension. This is a well-reported transfer and facilitates the ability to open up the first web space for key pinch.[22] Results can be improved with fusion of the distal radioulnar joint although this limits forearm rotation[24] and may also be augmented with PT to extensor pollicis longus tendon transfer.[45]
C7 and C7/8 injuries
C7 and C7/8 CSCIs benefit from patients having varying degrees of digital extension which allows surgeons to focus primarily on reconstructing finger flexion and intrinsic muscle function. As for high-level injuries, finger flexion may be best restored through nerve branch to brachialis transfer to AIN. In the absence of distal AIN innervation, it is recommended that intrinsic reinnervation is sought through distal nerve transfer of PIN branches to extensor indicis proprius (EIP), extensor pollicis brevis (EPB), and abductor pollicis longus (APL) to deep ulnar and recurrent median nerve fascicles, a transfer that has been reported in combined peripheral median and ulnar nerve injuries.[46],[47]
C8 injuries
For C8 CSCIs, the goal is a complete restoration of normal upper function. PIN branches to EIP, EPB, and APL can be transferred to recurrent median or deep ulnar nerve fascicles or AIN nerve branches to pronator quadratus can be transferred to deep ulnar nerve dependent on surgical preference. This recommendation considers C8 CSCIs essentially analogous to peripheral ulnar nerve injuries.[48],[49] Once again, there is no published literature demonstrating this transfer for CSCI, but reports from peripheral nerve injuries demonstrate improvements in grip strength and lateral pinch.
Conclusion
This paper presents a putative algorithm to guide reconstruction of upper limb function following CSCI, demonstrating that nerve transfer for functional reconstruction of CSCI is an area of hand surgery with very significant potential, but an evidence base limited to experimental case series and reports. In this context, the algorithm presented should not be perceived as a reconstructive protocol but as a provocative adjunct guided by functional priorities that should always be allied to the expertise of specialist surgeons. We encourage all colleagues with experience of nerve transfer in this highly specialist and deserving patient group to publish their experiences.
Acknowledgments
We thank the Warner Library, Mid Essex Hospital Services NHS Trust for support retrieving journal articles.
Ethical considerations
Ethical approval was not sought for this work.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Author's contributions
JW, DP jointly conceived the article, JW undertook the literature review, DP and JW jointly synthesized the reconstructive algorithm and wrote the manuscript. JW and DP jointly edited and reviewed the final manuscript and are responsible for the content and similarity index of the manuscript.
| 1. | NSCISC. Spinal Cord Injury (SCI) Facts and Figures at a Glance 2015 SCI Data Sheet. Available from: https://www.nscisc.uab.edu/Public/Facts%202015%20Aug.pdf. [Last accessed 2019 Jan14]. [Google Scholar] |
| 2. | DeVivo M, Chen Y, Mennemeyer S, Deutsch A. Costs of care following spinal cord injury. Top Spinal Cord Inj Rehabil Spring 2011;16:1-9. [Google Scholar] |
| 3. | French DD, Campbell RR, Sabharwal S, Nelson AL, Palacios PA, Gavin-Dreschnack D. Health care costs for patients with chronic spinal cord injury in the Veterans Health Administration. J Spinal Cord Med 2007;30:477-81. [Google Scholar] |
| 4. | Anderson KD, Fridén J, Lieber RL. Acceptable benefits and risks associated with surgically improving arm function in individuals living with cervical spinal cord injury. Spinal Cord 2009;47:334-8. [Google Scholar] |
| 5. | Hanson RW, Franklin MR. Sexual loss in relation to other functional losses for spinal cord injured males. Arch Phys Med Rehabil 1976;57:291-3. [Google Scholar] |
| 6. | Anderson KD. Targeting recovery: Priorities of the spinal cord-injured population. J Neurotrauma 2004;21:1371-83. [Google Scholar] |
| 7. | Snoek GJ, IJzerman MJ, Hermens HJ, Maxwell D, Biering-Sorensen F. Survey of the needs of patients with spinal cord injury: Impact and priority for improvement in hand function in tetraplegics. Spinal Cord 2004;42:526-32. [Google Scholar] |
| 8. | Senjaya F, Midha R. Nerve transfer strategies for spinal cord injury. World Neurosurg 2013;80:e319-26. [Google Scholar] |
| 9. | Cain SA, Gohritz A, Fridén J, van Zyl N. Review of upper extremity nerve transfer in cervical spinal cord injury. J Brachial Plex Peripher Nerve Inj 2015;10:e34-42. [Google Scholar] |
| 10. | Zhang S, Wang Y, Johnston L. Restoration of function in complete spinal cord injury using peripheral nerve rerouting: A summary of procedures. Surg Technol Int 2008;17:287-91. [Google Scholar] |
| 11. | Benassy J. A case of transposition of the musculo-cutaneous nerve upon the median nerve. Paraplegia 1965;3:199-202. [Google Scholar] |
| 12. | Kiwerski J. Recovery of simple hand function in tetraplegia patients following transfer of the musculo-cutaneous nerve into the median nerve. Paraplegia 1982;20:242-7. [Google Scholar] |
| 13. | Krasuski M, Kiwerski J. An analysis of the results of transferring the musculocutaneous nerve onto the median nerve in tetraplegics. Arch Orthop Trauma Surg 1991;111:32-3. [Google Scholar] |
| 14. | Bertelli JA, Tacca CP, Ghizoni MF, Kechele PR, Santos MA. Transfer of supinator motor branches to the posterior interosseous nerve to reconstruct thumb and finger extension in tetraplegia: Case report. J Hand Surg Am 2010;35:1647-51. [Google Scholar] |
| 15. | Bertelli JA, Ghizoni MF, Tacca CP. Transfer of the teres minor motor branch for triceps reinnervation in tetraplegia. J Neurosurg 2011;114:1457-60. [Google Scholar] |
| 16. | Brown JM. Nerve transfers in tetraplegia I: Background and technique. Surg Neurol Int 2011;2:121. [Google Scholar] |
| 17. | Fridén J, Gohritz A. Brachialis-to-extensor carpi radialis longus selective nerve transfer to restore wrist extension in tetraplegia: Case report. J Hand Surg Am 2012;37:1606-8. [Google Scholar] |
| 18. | Mackinnon SE, Yee A, Ray WZ. Nerve transfers for the restoration of hand function after spinal cord injury. J Neurosurg 2012;117:176-85. [Google Scholar] |
| 19. | Bertelli JA, Ghizoni MF. Transfer of nerve branch to the brachialis to reconstruct elbow extension in incomplete tetraplegia: Case report. J Hand Surg Am 2012;37:1990-3. [Google Scholar] |
| 20. | Bertelli JA, Mendes Lehm VL, Tacca CP, Winkelmann Duarte EC, Ghizoni MF, Duarte H. Transfer of the distal terminal motor branch of the extensor carpi radialis brevis to the nerve of the flexor pollicis longus: An anatomic study and clinical application in a tetraplegic patient. Neurosurgery 2012;70:1011-6. [Google Scholar] |
| 21. | Bertelli JA, Ghizoni MF. Single-stage surgery combining nerve and tendon transfers for bilateral upper limb reconstruction in a tetraplegic patient: Case report. J Hand Surg Am 2013;38:1366-9. [Google Scholar] |
| 22. | van Zyl N, Hahn JB, Cooper CA, Weymouth MD, Flood SJ, Galea MP. Upper limb reinnervation in C6 tetraplegia using a triple nerve transfer: Case report. J Hand Surg Am 2014;39:1779-83. [Google Scholar] |
| 23. | Davidge K, Kahne L, Novak CB, Juknis N, Ruvinskaya R, Fox I. Restoring prehension/ wrist flexion and decreasing spasticity 11 years following spinal cord injury: a case study of use of brachialis nerve transfer. American Society for Peripheral Nerve Annual Meeting; Hawaii, 2014. [Google Scholar] |
| 24. | Bertelli JA, Ghizoni MF. Nerve transfers for elbow and finger extension reconstruction in midcervical spinal cord injuries. J Neurosurg 2015;122:121-7. [Google Scholar] |
| 25. | Fox IK, Davidge KM, Novak CB, Hoben G, Kahn LC, Juknis N, et al. Use of peripheral nerve transfers in tetraplegia: Evaluation of feasibility and morbidity. Hand (N Y) 2015;10:60-7. [Google Scholar] |
| 26. | Hawasli AH, Chang J, Reynolds MR, Ray WZ. Transfer of the brachialis to the anterior interosseous nerve as a treatment strategy for cervical spinal cord injury: Technical note. Global Spine J 2015;5:110-7. [Google Scholar] |
| 27. | Fox IK, Davidge KM, Novak CB, Hoben G, Kahn LC, Juknis N, et al. Nerve transfers to restore upper extremity function in cervical spinal cord injury: Update and preliminary outcomes. Plast Reconstr Surg 2015;136:780-92. [Google Scholar] |
| 28. | Bertelli JA, Ghizoni MF. Nerve transfer for sensory reconstruction of C8-T1 dermatomes in tetraplegia. Microsurgery 2016;36:637-41. [Google Scholar] |
| 29. | Bertelli JA, Ghizoni MF. Nerve and free gracilis muscle transfers for thumb and finger extension reconstruction in long-standing tetraplegia. J Hand Surg Am 2016;41:e411-6. [Google Scholar] |
| 30. | Bertelli JA, Ghizoni MF. Nerve transfers for restoration of finger flexion in patients with tetraplegia. J Neurosurg Spine 2017;26:55-61. [Google Scholar] |
| 31. | Emamhadi M, Andalib S. Double nerve transfer for restoration of hand grasp and release in C7 tetraplegia following complete cervical spinal cord injury. Acta Neurochir (Wien) 2018;160;11:2219-24. [Google Scholar] |
| 32. | Fox IK, Novak CB, Kahn LC, Mackinnon SE, Ruvinskaya R, Juknis N, et al. Using nerve transfer to restore prehension and grasp 12 years following spinal cord injury: A case report. Spinal Cord Ser Cases 2018;4:37. [Google Scholar] |
| 33. | Sananpanich K, Kraisarin J, Siriwittayakorn W, Tongprasert S, Suwansirikul S. Double motor nerve transfer for all finger flexion in cervical spinal cord injury: An anatomical study and a clinical report. J Hand Surg Am 2018;43:920-6. [Google Scholar] |
| 34. | Blaauw G, Sauter Y, Lacroix CL, Slooff AC. Hypoglossal nerve transfer in obstetric brachial plexus palsy. J Plast Reconstr Aesthet Surg 2006;59:474-8. [Google Scholar] |
| 35. | Al-Thunyan A, Al-Qattan MM, Al-Meshal O, Al-Husainan H, Al-Assaf A. Hemi-hypoglossal nerve transfer for obstetric brachial plexus palsy: Report of 3 cases. J Hand Surg Am 2015;40:448-51. [Google Scholar] |
| 36. | Malessy MJ, Hoffmann CF, Thomeer RT. Initial report on the limited value of hypoglossal nerve transfer to treat brachial plexus root avulsions. J Neurosurg 1999;91:601-4. [Google Scholar] |
| 37. | Bhandari PS, Deb P. Use of contralateral spinal accessory nerve for ipsilateral suprascapular neurotization in global brachial plexus injury: A new technique. J Neurosurg Spine 2016;24:186-8. [Google Scholar] |
| 38. | Tubbs RS, Mortazavi MM, Shoja MM, Loukas M, Cohen-Gadol AA. Contralateral spinal accessory nerve for ipsilateral neurotization of branches of the brachial plexus: A cadaveric feasibility study. J Neurosurg 2011;114:1538-40. [Google Scholar] |
| 39. | Tubbs RS, Pearson B, Loukas M, Shokouhi G, Shoja MM, Oakes WJ, et al. Phrenic nerve neurotization utilizing the spinal accessory nerve: Technical note with potential application in patients with high cervical quadriplegia. Childs Nerv Syst 2008;24:1341-4. [Google Scholar] |
| 40. | Wang C, Yuan W, Zhou XH, Shi S, Wang X. Neurotization of the phrenic nerve with accessory nerve: A new strategy for high cervical spinal cord injury with respiratory distress. Med Hypotheses 2011;76:564-6. [Google Scholar] |
| 41. | Bertelli JA, Tacca CP, Winkelmann Duarte EC, Ghizoni MF, Duarte H. Transfer of axillary nerve branches to reconstruct elbow extension in tetraplegics: A laboratory investigation of surgical feasibility. Microsurgery 2011;31:376-81. [Google Scholar] |
| 42. | Leechavengvongs S, Teerawutthichaikit T, Witoonchart K, Uerpairojkit C, Malungpaishrope K, Suppauksorn S, et al. Surgical anatomy of the axillary nerve branches to the deltoid muscle. Clin Anat 2015;28:118-22. [Google Scholar] |
| 43. | Cricenti SV, Deangelis MA, Didio LJ, Ebraheim NA, Rupp RE, Didio AS, et al. Innervation of the extensor carpi radialis brevis and supinator muscles: Levels of origin and penetration of these muscular branches from the posterior interosseous nerve. J Shoulder Elbow Surg 1994;3:390-4. [Google Scholar] |
| 44. | Branovacki G, Hanson M, Cash R, Gonzalez M. The innervation pattern of the radial nerve at the elbow and in the forearm. J Hand Surg Br 1998;23:167-9. [Google Scholar] |
| 45. | Abrams GD, Ward SR, Fridén J, Lieber RL. Pronator teres is an appropriate donor muscle for restoration of wrist and thumb extension. J Hand Surg Am 2005;30:1068-73. [Google Scholar] |
| 46. | Power D, Nassimizadeh M, Samson D. Response to “Direct radial to ulnar nerve transfer to restore intrinsic muscle function in combined proximal median and ulnar nerve injury: Case report and surgical technique”. J Hand Surg Am 2015;40:858. [Google Scholar] |
| 47. | Phillips BZ, Franco MJ, Yee A, Tung TH, Mackinnon SE, Fox IK. Direct radial to ulnar nerve transfer to restore intrinsic muscle function in combined proximal median and ulnar nerve injury: Case report and surgical technique. J Hand Surg Am 2014;39:1358-62. [Google Scholar] |
| 48. | Novak CB, Mackinnon SE. Distal anterior interosseous nerve transfer to the deep motor branch of the ulnar nerve for reconstruction of high ulnar nerve injuries. J Reconstr Microsurg 2002;18:459-64. [Google Scholar] |
| 49. | Brown JM, Yee A, Mackinnon SE. Distal median to ulnar nerve transfers to restore ulnar motor and sensory function within the hand: Technical nuances. Neurosurgery 2009;65:966-77. [Google Scholar] |
Fulltext Views
2,028
PDF downloads
408





