Translate this page into:
Successful outcome of adjuvant denosumab therapy in treating unresectable spinal aneurysmal bone cyst in a pediatric patient: A case report and literature review
*Corresponding author: Fawaz N. Alshaalan, MD. Department of Spine Surgery, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia. fawaznalshaalan@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: AlRushud MA, Alshaalan FN, Konbaz FM, Aldebeyan SA. Successful outcome of adjuvant denosumab therapy in treating unresectable spinal aneurysmal bone cyst in a pediatric patient: A case report and literature review. J Musculoskelet Surg Res. doi: 10.25259/JMSR_72_2025
Abstract
Aneurysmal bone cysts (ABCs) are benign bone lesions that account for approximately 15% of all spine tumors. We present a case of an 8-year-old girl with a recurrent ABC of the cervical spine at the level of C3. She presented to the emergency department with myelopathy and weakness in her upper extremities 3 months after her initial surgery, which was a C3 corpectomy and fusion with iliac crest autograft followed by posterior decompression, tumor debulking, and instrumentation. The patient was not a candidate for complete surgical resection of the mass due to its encasement of the vertebral arteries. She underwent revision surgery with posterior marginal tumor resection and decompression of the cord. She then received adjuvant denosumab and demonstrated promising results, which were evident on computed tomography and magnetic resonance imaging as soon as 6 months post-denosumab treatment. This report also reviews the literature on treating ABCs and using adjuvant denosumab in the pediatric population.
Keywords
Aneurysmal
Bone
Cervical
Cyst
Spine
INTRODUCTION
Aneurysmal bone cysts (ABCs) are benign bone lesions that can either occur primarily or secondary to another lesion.[1-3] ABCs account for 1% of primary bone tumors, with an annual occurrence rate of 1.4 cases/100,000 individuals.[1,3] The majority of cases are observed during the first two decades of life.[1,3] The most common site for ABCs is the metaphysis of long bones, particularly around the knee. However, around one-third of these tumors originate in the spine, accounting for approximately 15% of all primary spine tumors, with the posterior elements being the most affected.[1,3]
Spinal ABCs typically manifest with pain, deformity, and/or neurological dysfunction secondary to swelling or mass effect.[1] On radiographs and computed tomography (CT), ABCs appear as expansile areas of bone loss with thin, dense borders.[1] On magnetic resonance imaging (MRI), these cysts are divided by connective tissue and bone partitions, with emphasized fluid levels and surrounding areas of increased enhancement.[1] Angiography displays poor vascularization of the cyst, while bone scans show heightened uptake at the periphery “doughnut sign.”[1,2]
The mainstay treatment of ABCs is surgical resection in conjunction with embolization to minimize blood loss during surgery. However, it has been reported that arterial embolization can be sufficient as a standalone treatment in some cases.[1] Recent studies suggest that denosumab can also be used for adjuvant treatment of ABCs alongside surgery and embolization or even as a standalone therapy in skeletally mature patients with spinal ABCs.[4]
Denosumab has proven its efficacy in treating a multitude of pathologies such as osteoporosis, bone metastases, and fibrous dysplasia, and more recently, it has gained interest in treating giant cell tumors (GCT) of the bone.[5] Due to the many similarities between GCT and ABC, it has been hypothesized that it can also be used to treat ABCs. However, the literature is scarce in this regard, and no randomized controlled trials have been conducted to demonstrate its efficacy in treating ABCs.[4] In this paper, we present a case of a recurrent spinal ABC in a pediatric patient, which was treated with revision surgical resection and adjuvant denosumab therapy.
CASE PRESENTATION
An 8-year-old Middle Eastern girl presented to our ER with a sudden onset of weakness and paresthesia in her upper extremities. She provided a history of ABC at the level of C3 that was confirmed by biopsy. Three months before her presentation in our emergency room (ER), she underwent a front-back procedure, including a C3 corpectomy, iliac crest bone grafting, and anterior plating, followed by a posterior partial resection of the tumor and instrumentation from C2 to C5. Her initial surgery was done at another hospital.
Clinical findings
The patient presented to our ER ambulatory, not in any distress. On examinations, she had bilateral weakness in shoulder abduction that was worse on the left side. She also had bilateral upper limb paresthesia. Her American Spinal Injury Association (ASIA) score was C.[6] During the examination of upper motor neuron signs, she had positive Hoffman’s and sustained clonus.
Radiographs showed a kyphotic deformity in the cervical spine in addition to the posterior and anterior fixation that the patient underwent at her local hospital [Figure 1]. An urgent MRI of the cervical spine with IV contrast was done, which revealed a residual/recurrent disease process and post-surgical changes with heterogeneous areas of enhancement representing septations with multiple fluid-fluid levels causing severe cord compression and myelomalacia. The lesion completely encased the vertebral arteries bilaterally at the level of C3 [Figure 2]. The patient was started on a dexamethasone loading dose, then maintenance, and booked for urgent selective arterial embolization. The tumor received numerous feeders from the occipital, ascending cervical, deep cervical, and vertebral arteries bilaterally. The embolization process was labeled limited by the Interventional Radiology team as they were only able to embolize three feeders from the right ascending, right deep cervical, and left occipital artery. Most tumor feeders had angiographically gross dangerous collateral connections to the vertebral arteries that were not embolized out of fear of occluding the vertebral arteries. Pre-operative CT was performed and showed stable implants with a mild kyphotic deformity in the cervical spine [Figure 3].
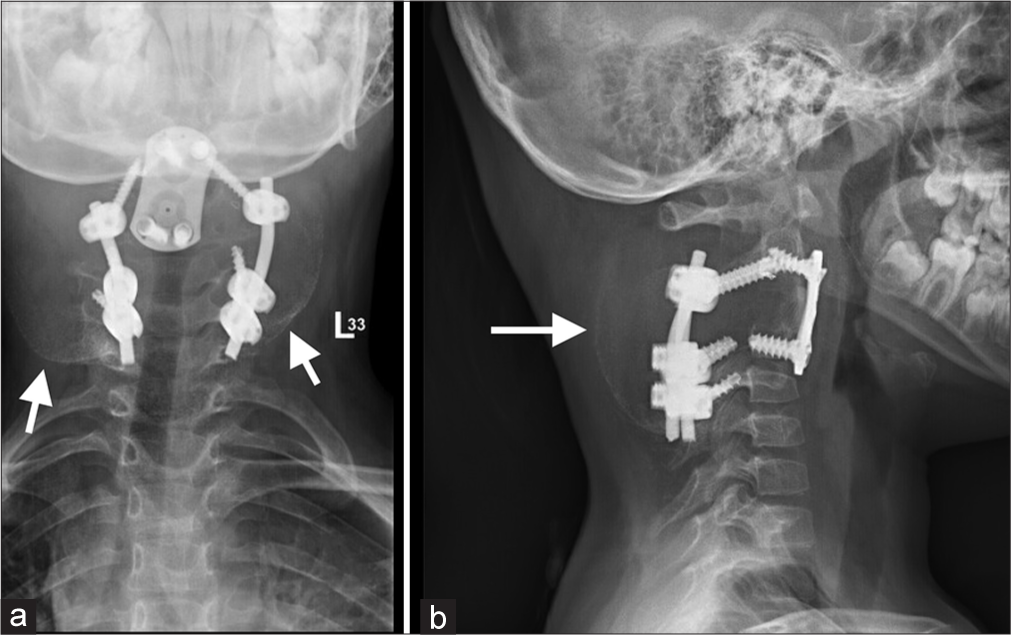
- (a) Anteroposterior and (b) Lateral radiographs on presentation showing previous hardware with arrows pointing the expansile lesion involving the upper cervical spine.
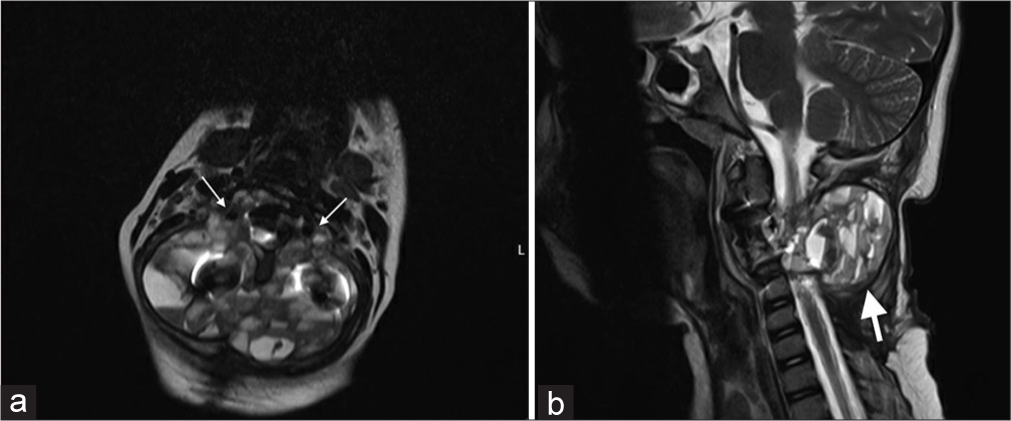
- (a) Axial and (b) Sagittal magnetic resonance imaging on presentation shows the lesion at C3, arrows showing the tumor encasing the vertebral artery bilaterally.
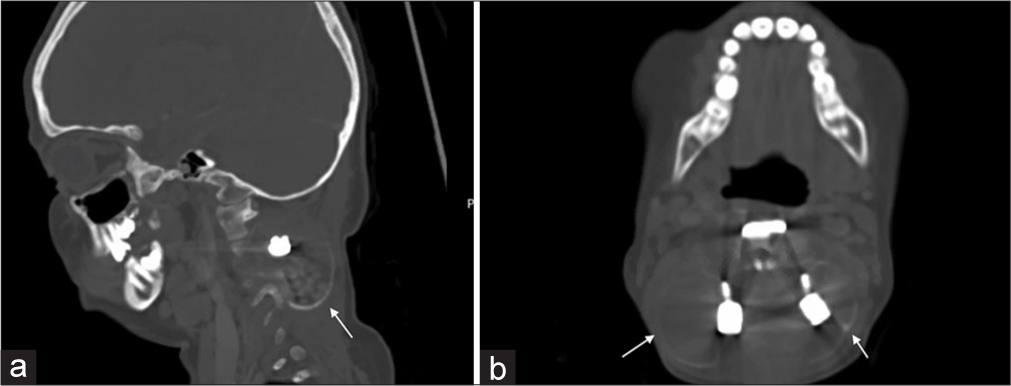
- (a) Sagittal and (b) Axial computed tomography on presentation shows the extent of the lesion (arrows) with stable implants and a mild kyphotic deformity in the cervical spine.
Surgical intervention
The patient was taken to the operating room for urgent decompression through a posterior approach. Intraoperative findings were consistent with imaging. Instrumentation was found to be stable and complete resection was deemed impossible due to complete bilateral encasement of the vertebral arteries by the lesion. Therefore, marginal resection of the tumor with decompression of the cord was carried out, and a biopsy was sent, which confirmed the diagnosis of ABC.
Three weeks postoperatively, the patient recovered fully with an ASIA score of E.[6] The medical oncology team was involved and started the patient on denosumab therapy 2 months after surgery. The regimen was 70 mg/m2/dose, equaling 60 mg SC weekly for 4 weeks, followed by once monthly for 12 months with supplemental Vitamin D and calcium carbonate. Biochemistry was assessed monthly and remained stable except for a few episodes of mild hypocalcemia that required supplement dose adjustment. A follow-up CT was done 12 months after treatment initiation and showed near-complete ossification of the residual expansile osseous lesion [Figure 4]. The patient completed her denosumab therapy course without any complications. The patient remained neurologically intact without complications during her 2-year follow-up visit.
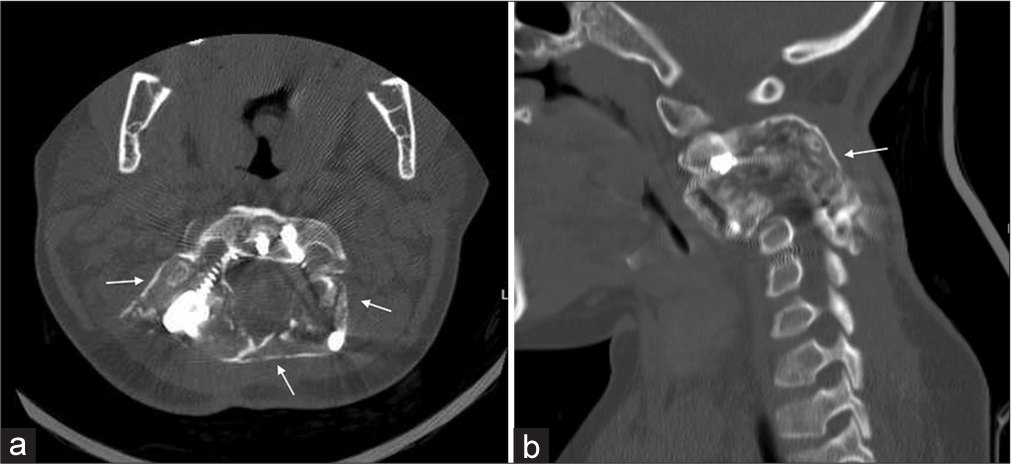
- Follow-up (a) Axial and (b) Sagittal computed tomography with arrows showing complete calcification and fusion of the lesion.
LITERATURE REVIEW
Methods
We utilized online databases such as Medline, Web of Science Core Collection, and Google Scholar for article collection. Our literature review was limited to articles published from 2010 and onwards. Since Denosumab was Food and Drug Administration (FDA)-approved in June 2010 and only for postmenopausal osteoporosis, we saw no need to extend our research further.[7] All articles that reported the use of denosumab for the treatment of ABC were isolated and then narrowed down further to spinal/peri-spinal ABCs. Publications without radiological follow-up were excluded from this study. All collected papers were either case reports or case series. Each subject reported in a case series was reported separately in our paper.
We identified 14 articles with cases of ABCs in the spine, sacrum, or costovertebral angle treated with denosumab. From these 14 articles, we identified 28 subjects, five pediatric and 23 adults, who underwent medical treatment for spinal ABC with denosumab. Out of those 28, 16 patients received denosumab as a first-line treatment due to surgery being too risky or associated with high morbidity. One of the 16 patients underwent curettage and bone grafting after 4 months of denosumab therapy, while the rest did not undergo any other interventions. Out of the remaining 12 patients, eight underwent surgical resection, three underwent embolization, and one patient underwent chemo and radiotherapy before starting denosumab as she was initially misdiagnosed as a case of osteosarcoma [Supplementary Table 1].[8-23]
RESULTS
In all cases but two, symptomatic improvement was reported [Supplementary Table 1]. Only two cases reported no change in patients’ symptoms.[8,9] All papers reported radiological improvement after treatment, which was evident by ossification and/or tumor shrinkage on imaging on follow-up. Recurrence was reported in five cases; however, follow-up ranged between 2 and 37 months following treatment, which could affect the reported recurrence rate as the earliest reported recurrence happened 10 months after discontinuation of denosumab therapy.[10,11] There was no long-term follow-up in any of the papers that we found.
Furthermore, the denosumab regimen and duration of treatment varied between papers, which could alter the outcome and recurrence rate. Overall, denosumab seems to have a relatively safe profile in the treatment of ABCs, as there were only two cases of severe rebound hypercalcemia following discontinuation of treatment.[12,13] Other reported adverse effects were mild and included grade 1 vomiting, mild hypocalcemia during therapy, and mild hypercalcemia after discontinuation of treatment.[10,13]
DISCUSSION
ABCs of the spine can be challenging to treat, especially in a revision setting.[24] The mainstay treatment of ABCs is complete resection to decrease the chance of local recurrence.[24] When complete resection is deemed impossible, second-line treatment modalities become essential.[10] Partial resection and adjuvant therapy have been increasing in popularity as an alternative and are being reported in the literature with successful outcomes.[10] One of the popular adjuvants in treating ABCs is denosumab; however, its use in the pediatric population is mostly off-label.[10] Denosumab has been approved by the FDA for treating multiple pathologies in adults and skeletally mature adolescents. However, there are concerns regarding its safety in skeletally immature patients.[22] Complications such as growth disturbance and growth plate ossification have been reported in the literature.[22]
Furthermore, research on the safety of denosumab in skeletally immature patients is lacking, which hinders its approval by the FDA and, in turn, its widespread use.[22] Due to a lack of sizable data on using denosumab to treat ABCs, there is no consensus in the literature regarding dosage and length of therapy.[22] Most articles describe the same denosumab adult dose as the one used for GCT to be used for the treatment of spinal ABCs. However, the pediatric dose is more challenging as the drug is not FDA-approved to be used for any pediatric pathology [Supplementary Table 1].[22]
The clinical picture of spinal ABC differs from other sites, as patients can present with neurological symptoms alongside the expected pain and pathological fractures that are associated with ABCs in other musculoskeletal sites.[1] The main indication for using denosumab in our case was the rapid recurrence and difficulty in achieving complete surgical resection of the mass due to its bilateral encasement of the vertebral arteries. The degree of bilateral vertebral artery encasement was complete and categorized as D according to the Westbroek et al. classification.[25] Furthermore, the presence of a neurological deficit on examination constituted the urgency of surgical intervention in our case and excluded the option to treat with denosumab alone.
In our literature review, we identified three papers that reported a total of five pediatric patients with spinal ABC who underwent adjuvant denosumab therapy following surgical resection of their lesions [Supplementary Table 2].[10,13,19] It was reported that all five patients showed clinical and radiological improvement following the denosumab therapy with no serious side effects.[10,13,19] There was only a single case of recurrence at 10 months after denosumab therapy discontinuation.[13]
In all three articles, denosumab was utilized following recurrence.[10,13,19] Multiple interventions were considered or carried out in all patients, but one with no discernable improvement before starting denosumab therapy.[10,13,19] Fadavi et al. presented a case where denosumab was used after multiple interventions were carried out for repeated recurrence.[19] After denosumab treatment, the patient had no recurrences for over 12 months.[19] Not only was denosumab useful in preventing recurrence and stabilizing the lesion but also Raux et al. reported on the efficacy of denosumab in improving neurological dysfunction when used alone in cases of recurrence presenting with neurological deficits.[13]
The denosumab regimen differed between the three articles. Lange et al. suggested individualizing the regimen depending on the case.[10] The more critical patients received an induction dose of weekly injections for 1 month followed by monthly injections, while the more stable patients could be managed with monthly injections to minimize side effects.[10] In contrast, Raux et al. started all patients with weekly injections for 1 month, followed by monthly injections.[13] Regarding dosage, both Lange et al. and Raux et al. used 70 mg/m2, while Fadavi et al. used the GCT adult dose of 120 mg, which is also recommended for 13-year-olds weighing more than 45 kg.[10,13,19]
CONCLUSION
Denosumab has the potential to be an important adjuvant for spine surgeons in the treatment of ABCs. This is especially true in patients who undergo incomplete or marginal resections with remnant/recurrent disease. However, further research is warranted to illustrate the efficacy and safety of denosumab therapy in treating ABCs in pediatrics and to establish an ideal regimen that would consider the treatment’s risks and benefits.
Authors’ contributions
MAA: Data collection, data Analysis, and writing-original draft. FNA: Data collection, processing, and writing original draft. FMK: Data collection and analysis, reviewing, and editing. SAA: Data curation, methodology, validation, visualization, and project administration. All authors have critically reviewed and approved the final draft and are responsible for the manuscript’s content and similarity index.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient’s parents have given their consent for the patient’s images and other clinical information to be reported in the journal. The parents understand that the patient’s name and initials will not be published, and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Conflicts of interest
There are no conflicting relationships or activities.
Financial support and sponsorship: This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Spinal aneurysmal bone cysts (ABCs): Optimal management. Orthop Res Rev. 2019;11:159-66.
- [CrossRef] [PubMed] [Google Scholar]
- Modern surgical treatment of primary aneurysmal bone cyst of the spine in children and adolescents. J Pediatr Orthop. 2005;25(3):387-92.
- [CrossRef] [PubMed] [Google Scholar]
- Aneurysmal bone cyst of the spine: Report of four cases and review of the literature. Interdiscip Neurosurg. 2019;16:18-21.
- [CrossRef] [Google Scholar]
- The role of neoadjuvant denosumab in the treatment of aneurysmal bone cysts: A case series and review of the literature. J Neurosurg Pediatr. 2022;30:547-54.
- [CrossRef] [PubMed] [Google Scholar]
- Denosumab In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2025. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535388
- [Google Scholar]
- International standards for neurological classification of spinal cord injury: Revised 2019. Top Spinal Cord Inj Rehabil. 2021;27:1-22.
- [CrossRef] [PubMed] [Google Scholar]
- Denosumab in patients with aneurysmal bone cysts: A case series with preliminary results. Tumori. 2018;104:344-51.
- [CrossRef] [PubMed] [Google Scholar]
- Aneurysmal bone cyst: Results of an off label treatment with denosumab. BMC Musculoskelet Disord. 2019;20:456.
- [CrossRef] [PubMed] [Google Scholar]
- Denosumab: A potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J. 2013;22:1417-22.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy of denosumab in treating spinal aneurysmal bone cyst: A case report. Cureus. 2023;15:e39954.
- [CrossRef] [PubMed] [Google Scholar]
- Denosumab treatment in aneurysmal bone cyst: Evaluation of nine cases. Pediatr Blood Cancer. 2018;65:e26926.
- [CrossRef] [PubMed] [Google Scholar]
- Denosumab for treating aneurysmal bone cysts in children. Orthop Traumatol Surg Res. 2019;105:1181-5.
- [CrossRef] [PubMed] [Google Scholar]
- Response of aneurysmal bone cyst to denosumab. Spine (Phila Pa 1976). 2015;40:E1201-4.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting receptor-activator of nuclear kappaB ligand in aneurysmal bone cysts: Verification of target and therapeutic response. Transl Res. 2014;164:139-48.
- [CrossRef] [PubMed] [Google Scholar]
- Interest of denosumab for the treatment of giant-cells tumors and aneurysmal bone cysts of the spine. About nine cases. Spine (Phila Pa 1976). 2016;41:E654-60.
- [CrossRef] [PubMed] [Google Scholar]
- Denosumab: A potential new treatment option for recurrent aneurysmal bone cyst of the spine. SICOT J. 2019;5:10.
- [CrossRef] [PubMed] [Google Scholar]
- Denosumab: Non-surgical treatment option for selective arterial embolization resistant aneurysmal bone cyst of the spine and sacrum. Case report. Eur Rev Med Pharmacol Sci. 2016;20:3692-5.
- [Google Scholar]
- Dramatic response of aneurysmal bone cyst to denosumab: Case report and literature review. Clin Case Rep. 2021;9:e04993.
- [CrossRef] [PubMed] [Google Scholar]
- Aggressive osteoblastoma with a secondary aneurysmal bone cyst treated with denosumab. Rare Tumors. 2021;13:20363613211034710.
- [CrossRef] [PubMed] [Google Scholar]
- Denosumab: A potential treatment option for aneurysmal bone cyst of the atlas. Eur Spine J. 2018;27:494-500.
- [CrossRef] [PubMed] [Google Scholar]
- Denosumab in pediatric bone disorders and the role of RANKL blockade: A narrative review. Transl Pediatr. 2023;12:470-86.
- [CrossRef] [PubMed] [Google Scholar]
- Denosumab role in sacral aneurysmal bone cyst: A rare case report. IP Int J Med Paediatr Oncol. 2019;5:108-11.
- [CrossRef] [Google Scholar]
- Denosumab in the management of aneurysmal bone cyst. Joint Bone Spine. 2022;89:105260.
- [CrossRef] [PubMed] [Google Scholar]
- Vertebral artery sacrifice versus skeletonization in the setting of cervical spine tumor resection: Case series. World Neurosurg. 2020;139:e601-7.
- [CrossRef] [PubMed] [Google Scholar]







