Translate this page into:
Interim analysis of recruitment data for a randomized control trial of digital nerve repair
Corresponding Author:
Sunil Parthiban
Hand and Peripheral Nerve Unit, Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham B15 2TH
United Kingdom
sunil.parthiban@uhb.nhs.uk
| How to cite this article: Parthiban S, Foster MA, Beale S, Power DM. Interim analysis of recruitment data for a randomized control trial of digital nerve repair. J Musculoskelet Surg Res 2019;3:86-89 |
Abstract
Objectives: Digital nerves provide a good model for testing efficacy of repair techniques. Injuries are often standardized, with high numbers of patients and reasonable follow-up time scales. The gold standard treatment for a complete, traumatic peripheral nerve lesion involves direct end-to-end microsurgical repair. Conduits may reduce tethered scar at repair sites and provide a supported segment for a sutureless repair. A randomized controlled trial (RCT) investigating the outcomes of digital nerve repair requires a two-stage recruitment process. Interim analysis of our study design aimed to identify the areas of improvement for recruitment efficiency. Methods: Patients with reduced sensation in a digital nerve distribution were referred to a research nurse for consent and first-stage recruitment to the Conduit Nerve approximation versus Neurorrhaphy Evaluation of Clinical outcome Trial (CoNNECT). Intraoperative confirmation of a complete nerve injury allowed second-stage recruitment and randomization. Analysis of screening data and recruitment logs from June 2017 to December 2018 enabled the assessment of recruitment efficiency. Results: We assessed 268 patients as suitable for CoNNECT with 82% consenting to take part in the trial. Eighty-five patients were deemed suitable intraoperatively; however, only 69 patients were successfully recruited. Patients were missed due to operating surgeons not being trained in the CoNNECT protocol, time constraints, and inadequate planning of theater resources. Conclusion: Key areas effecting recruitment include adequate provision of staff training workshops, prioritization of trial patients, and improved communication with patients. The results of this audit into recruitment to an RCT in digital nerve repair will help inform future studies in this area.Introduction
Recruitment for a randomized controlled trial (RCT) is difficult to predict, and poor planning of a study can lead to disappointing recruitment rates. Walters et al. analyzed 151 publicly funded RCTs over 12 years and noted that only 56% of those trials were able to achieve their target recruitment.[1] This figure is similar to that from Sully et al.,[2] who found that 55% of trials achieved their target recruitment between 2002 and 2008. Similarly, there are difficulties with retention of patients during a trial. Walters et al.[1] found that 79%–97% of participants had valid primary outcome data after the study closed.
There are numerous steps in a patient's trial pathway: the initial screening and consent and the intervention and subsequent follow-ups. Walters et al.[1] found that only 70% of patients deemed eligible at screening were consented and randomized. This brief study outlines our experiences with recruitment and some of the challenges faced in recruiting to an RCT.
Materials and Methods
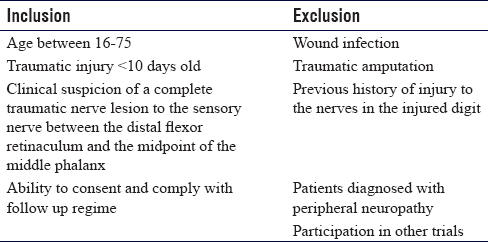
Our center is currently conducting an investigator-led, commercially funded RCT. Conduit Nerve approximation versus Neurorrhaphy Evaluation of Clinical outcome Trial (CoNNECT) aims to investigate the outcomes of patients who have had a digital nerve injury with subsequent repair. We aim to collect the data from 240 nerve injuries, based on our power analysis and predicted dropout rate of 30%. Patients are initially assessed in our acute hand clinic. If they met preoperative eligibility [Table - 1], they were offered to take part in the trial. Patients found to have a complete nerve injury intraoperatively were randomized to one of the three arms in the study. All patients had their assessments stored on our electronic database with their operation notes. Ethical approval was sought and granted by West MidlandsSolihull Research Ethics Committee (17/WM/0009). Written informed consent was obtained from all participants before recruitment. The trial specifically evaluates a detensioning, sutureless co-aptation using the Neurolac® (Polyganics, Netherlands) alone versus a standard microsurgical repair, or a microsurgical repair with a Neurolac®. The trial is supported by a commercial grant from Polyganics.
Screening log data were collected for all patients from June 17, 2017, to December 2, 2018. Hospital numbers were used to identify and extract clinical assessment data and operative records from an electronic database generated contemporaneously through clinical data entry in an electronic patient record system (eHands) developed at our center. Injury demographics including the anatomical site of injury, concomitant injuries, and intraoperative findings were included for analysis.
Results
Following presentation to the acute hand injury clinic and completion of the initial clinical assessment, interrogation of the eHands database identified 268 patients who met the first-stage preoperative eligibility criteria for potential recruitment to the CoNNECT study. After discussion of the trial design and provision of a CoNNECT study patient information sheet, 219/268 (82%) patients consented to study participation. Confirmation of eligibility was made after surgical exploration of the traumatic wound and 85/219 (39%) met the intraoperative inclusion criteria for the study. [Figure - 1] shows a flowchart of the trial pathway from assessment to randomization.
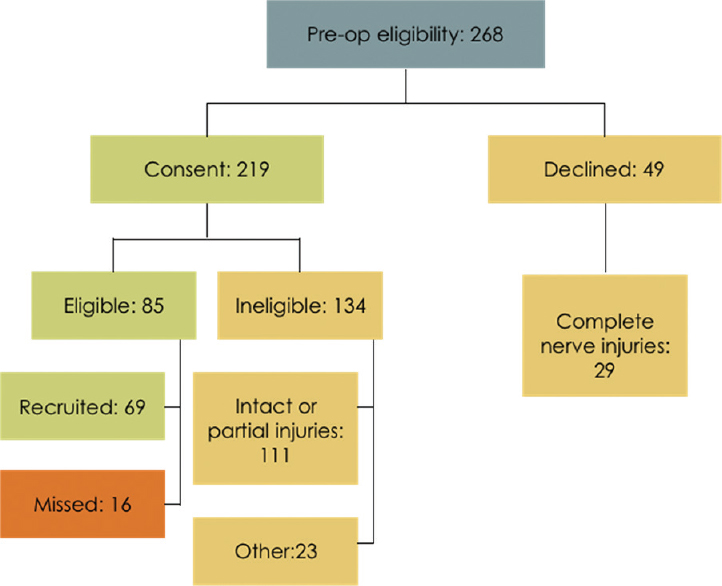 |
| Figure 1: Flowchart of our trial pathway from screening to operation. 82% consented to trial participation and 18% declined |
Fifty patients had multiple nerve injuries identified after wound exploration. There were 106 nerve injuries identified intraoperatively from the 85 eligible patients (1.24 nerves/patient). Not all patients with intraoperative confirmation of eligibility were recruited to the study; 69 patients (81 nerves) have been recruited to the study to date. Sixteen patients with 25 nerve injuries (1.56 nerves/patient) were missed for second-stage recruitment and randomization for a number of reasons as demonstrated in [Figure - 2]. Of the 16 missed patients, one was missed due to the patient being taken to theater before full trial consent could take place, a second was missed due to a communication error with the research nursing team for randomization during the procedure, and ten nerves were missed because of inadequate theater time in a complex injury. Other reasons for failure to randomize included delay time to surgery resulting in failure to meet the trial eligibility criteria, the operating surgeon not being trained in the trial protocol, and polytrauma.
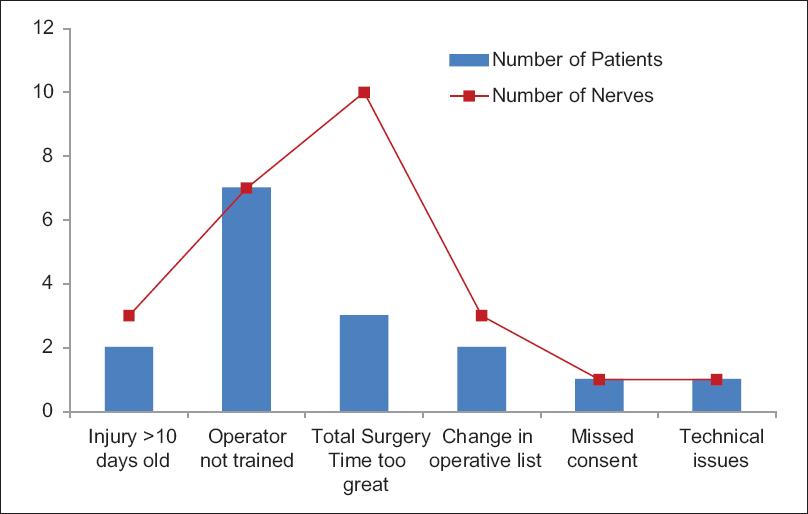 |
| Figure 2: Chart showing why patients were not recruited to the trial despite being eligible. Blue bars represent patient numbers and red squares represent number of eligible nerve injuries that were not recruited. Some patients had multiple nerve injuries, particularly when operative time was the reason for nonrecruitment |
A total of 49 patients declined to take part in the study after passing the first-stage eligibility discussion and provision of a patient information sheet. There were a total of 29 complete nerve injuries that would have been eligible for the trial in this subset.
Of the 69 recruited patients, there were 32 left-hand injuries and 37 right-hand injuries. The location of nerve injuries is shown in [Table - 2]. Some patients had an injury at the level of the common digital nerve, which had a specific digit mentioned in the operation note. For these patients, we have included the recorded digit in the table. Where a common digital nerve is injured, both digits are recorded in the anatomical dataset. Some patients had digital nerve injuries to both radial digital nerve (RDN) and ulnar digital nerves (UDNs) in the same digit. We have counted these as one finger in the table for data analysis.
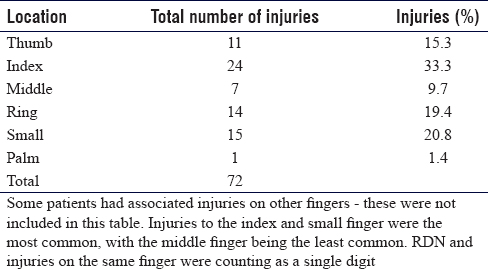
[Figure - 3] shows the location of nerve injury to either palmar or digit injuries. Eleven patients had an isolated nerve injury of the RDN or the UDN. An isolated injury was defined as one where only a single digital nerve was injured with no other injured structure within the digit. The RCT we are conducting does not include those with distal digital nerve injuries beyond the mid-point of the middle phalanx. Of the 11 isolated injuries, three were of the thumb, four from the index, one from the ring, and three from the small finger. There were no isolated middle finger digital nerve injuries.
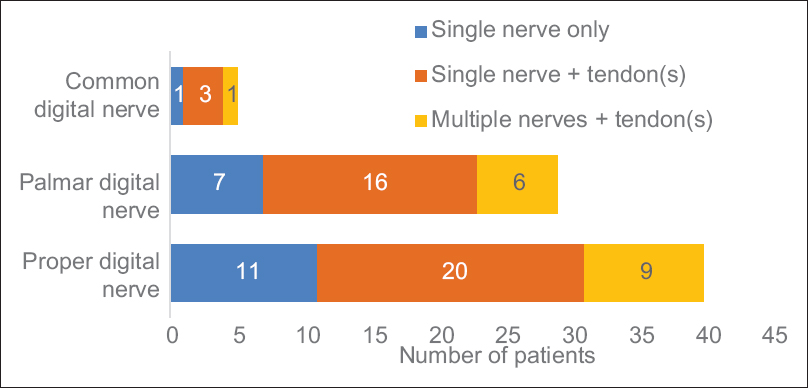 |
| Figure 3: Total number of patients grouped by location of injury. Isolated injuries are those that have no other injury in the same or contralateral hand. There will be overlapping of figures in the yellow groups as some patients had both palm + ulnar digital nerve and radial digital nerve injuries. Twenty patients had a single digital nerve injury with a tendon injury in the same hand. Nine patients had multiple nerve and tendon injuries |
Including those with associated injuries, there were 20 additional patients with a digital nerve injury. Nine of those patients had digital nerve injuries on multiple fingers and one patient having an injury to both the radial and UDN on the same finger.
Discussion
This audit of trial recruitment to a single-center RCT in digital nerve repair (CoNNECT) has helped identify injury patterns, associated injuries, and recruitment challenges. The demographic data on injury distribution will be useful for researchers developing trials in digital nerve repair and will assist in feasibility studies and provide guidance on predicted recruitment duration. The results of this audit have identified several factors that have influenced trial recruitment in our study and have provided an opportunity for targeting resources to increase recruitment rate. These areas include junior doctor examination and consent training, surgeon training workshops, research support staff availability, clinical and research team integration, and trial awareness within the study population.
Clinical research is a key part of junior doctor development.[3] Several reports in the literature have shown that successful recruitment to major trials requires engagement from clinicians with positive relationships between research and clinical teams.[4],[5] Our experiences have been similar, and to that end, we have integrated our research team to our daily clinical practice, ensuring that both teams are easily accessible to each other. Monthly departmental meetings are held where a research update is given to all clinicians to reinforce the research aims of the department and provide regular recruitment updates.
Paramasivan et al.[6] identified recruiter bias, poor communication, and lack of awareness of the trial as a key challenge for their study recruitment. This has been similarly echoed in other studies.[7],[8],[9],[10] We run frequent education sessions for junior clinicians to help them understand the trial aims and the protocol requirements to help address this issue. However, the high turnover of doctors on rotating placements requires continuous recruitment training workshops to ensure that all trainees are aware of the trial.
Walters et al.[1] found that consent rate was approximately 70% in their systematic review of recruitment and retention rates in RCTs in the United Kingdom. This is lower than the CoNNECT consent rate to date, which is most likely related to the requirement for a two-staged consent process. The patient is informed about the trial at first contact when there is a suspicion of a nerve injury based on the clinical examination. At this point, they are provided with a patient information sheet and the majority of patients are booked for an urgent day case attendance for their surgery, with a formal trial consent being taken at that second visit. There is a low recruitment rate (26%) across the whole study, possibly due to the rigorous inclusion criteria and the poor correlation between altered sensation on clinical examination after injury and the presence of a complete nerve transection injury. Assessing a digital nerve preoperatively is difficult, with one study showing an 8.3%–14% error rate.[11] A high definition ultrasound may help identify digital nerve transections; however, most studies have been aimed toward.
During this audit, there were seven patients who were not recruited at operation despite appropriate trial consent due to the lead operating surgeon not having completed a training workshop for the trial. This has been seen as a common reason for recruitment failure in other studies as well.[12],[13]
Methods to address this include frequent education programs for clinicians within the team and the use of practical surgical training workshops to demonstrate the three study arms, to confirm competence, and to allow new rotating surgeons to be approved to undertake trial procedures in the delegation log. Tackling preventable causes of recruitment failure continues to be a major part of trial management. Studies have shown that lack of understanding from clinicians continues to be a large contributor to missed patients.[7],[14],[15]
Conclusion
Recruitment to RCTs is a complex process with many factors contributing to the success and efficiency of the trial. Analysis of this CoNNECT audit data has provided useful demographic information on presentation, site, and severity of digital nerve injuries and common associated injuries. The audit has identified areas for improvement where the research team can focus resources to improve recruitment. Key areas we have identified include adequate provision of staff training workshops, prioritization of trial patients to meet inclusion criteria, and improved communication with patients. When designing trials, we would emphasize the importance of early clinician consultation and engagement. The results of this audit into recruitment to an RCT in digital nerve repair will help inform future studies in this area.
Ethical approval
Ethical approval was sought and granted by West Midlands –Solihull Research Ethics Committee (17/WM/0009).
Financial support and sponsorship
The CoNNECT study is supported by a commercial grant from Polyganics.
Conflicts of interest
There are no conflicts of interest.
Author's contributions
DMP conceived the aims of this audit and gave input into its final draft and provided logistical support. SP analyzed and interpreted the data and wrote the initial and final draft. MAF gave input into the final draft. SB was involved with collection of the data and implementation of the study. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Walters SJ, Bonacho dos Anjos Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, et al. Recruitment and retention of participants in randomised controlled trials: A review of trials funded and published by the United Kingdom health technology assessment programme. BMJ Open 2017;7:e015276. [Google Scholar] |
| 2. | Sully BG, Julious SA, Nicholl J. A reinvestigation of recruitment to randomised, controlled, multicenter trials: A review of trials funded by two UK funding agencies. Trials 2013;14:166. [Google Scholar] |
| 3. | Kasivisvanathan V, Tantrige PM, Webster J, Emberton M. Contributing to medical research as a trainee: The problems and opportunities. BMJ 2015;350:h515. [Google Scholar] |
| 4. | Borschmann R, Patterson S, Poovendran D, Wilson D, Weaver T. Influences on recruitment to randomised controlled trials in mental health settings in England: A national cross-sectional survey of researchers working for the Mental Health Research Network. BMC Med Res Methodol 2014;14:23. [Google Scholar] |
| 5. | Peckham E, Arundel C, Bailey D, Callen T, Cusack C, Crosland S, et al. Successful recruitment to trials: Findings from the SCIMITAR+trial. Trials 2018;19:53. [Google Scholar] |
| 6. | Paramasivan S, Huddart R, Hall E, Lewis R, Birtle A, Donovan JL, et al. Key issues in recruitment to randomised controlled trials with very different interventions: A qualitative investigation of recruitment to the SPARE trial (CRUK/07/011). Trials 2011;12:78. [Google Scholar] |
| 7. | Howard L, de Salis I, Tomlin Z, Thornicroft G, Donovan J. Why is recruitment to trials difficult? An investigation into recruitment difficulties in an RCT of supported employment in patients with severe mental illness. Contemp Clin Trials 2009;30:40-6. [Google Scholar] |
| 8. | Rooshenas L, Elliott D, Wade J, Jepson M, Paramasivan S, Strong S, et al. Conveying equipoise during recruitment for clinical trials: Qualitative synthesis of clinicians' practices across six randomised controlled trials. PLoS Med 2016;13:e1002147. [Google Scholar] |
| 9. | Donovan JL, Paramasivan S, de Salis I, Toerien M. Clear obstacles and hidden challenges: Understanding recruiter perspectives in six pragmatic randomised controlled trials. Trials 2014;15:5. [Google Scholar] |
| 10. | Elliott D, Husbands S, Hamdy FC, Holmberg L, Donovan JL. Understanding and improving recruitment to randomised controlled trials: Qualitative research approaches. Eur Urol 2017;72:789-98. [Google Scholar] |
| 11. | Dehghani M, Shemshaki H, Eshaghi MA, Teimouri M. Diagnostic accuracy of preoperative clinical examination in upper limb injuries. J Emerg Trauma Shock 2011;4:461-4. [Google Scholar] |
| 12. | Briel M, Olu KK, von Elm E, Kasenda B, Alturki R, Agarwal A, et al. Asystematic review of discontinued trials suggested that most reasons for recruitment failure were preventable. J Clin Epidemiol 2016;80:8-15. [Google Scholar] |
| 13. | Watson JM, Torgerson DJ. Increasing recruitment to randomised trials: A review of randomised controlled trials. BMC Med Res Methodol 2006;6:34. [Google Scholar] |
| 14. | Abraham NS, Young JM, Solomon MJ. A systematic review of reasons for nonentry of eligible patients into surgical randomized controlled trials. Surgery 2006;139:469-83. [Google Scholar] |
| 15. | Ziebland S, Featherstone K, Snowdon C, Barker K, Frost H, Fairbank J, et al. Does it matter if clinicians recruiting for a trial don't understand what the trial is really about? Qualitative study of surgeons' experiences of participation in a pragmatic multi-centre RCT. Trials 2007;8:4. [Google Scholar] |
Fulltext Views
2,689
PDF downloads
1,565





