Translate this page into:
A comparison of tendon and nerve transfer surgery for reconstruction of upper limb paralysis
2 Department of Orthopaedic Surgery, Royal Orthopaedic Hospital, Birmingham, UK
Corresponding Author:
Davina C Cavallaro
Queen Elizabeth Hospital, Birmingham
UK
dcavallaro@nhs.net
| How to cite this article: Cavallaro DC, Mikalef P, Power DM. A comparison of tendon and nerve transfer surgery for reconstruction of upper limb paralysis. J Musculoskelet Surg Res 2019;3:69-74 |
Abstract
Paralysis of the upper limb muscles may follow peripheral nerve injury, spinal cord injury, nerve compression, tumour resection, inflammatory neuropathy, spot-infective neuritis or as a result of a degenerative neurological disease. The goals of treatment are to restore important functions without losing important donor muscle function. Caution should be exerted when the underlying process is a progressive one because initially successful reconstruction may be followed by a delayed deterioration. Tendon transfer and nerve transfer surgery are two of the reconstructive techniques available for functional restoration of paralysis. Tendon transfer redirects a muscle-tendon unit for a more critical function losing some muscle power in the process. Nerve transfer uses an expendable donor nerve branch or fascicle from within a nerve trunk to re-innervate the paralysed muscle in its original bed. These procedures may be combined either with functioning free muscle transfers or with arthrodesis procedures to stabilise important joints and free up additional muscle-tendon or nerves for transfer. Hybrid reconstruction with combined tendon transfer and nerve transfer may achieve greater potential gains for an individual than either technique in isolation. We aim to provide the reader with a broad overview of the above techniques to better inform decision-making when faced with a patient with functional deficit of the upper limb.
Introduction
The restoration of function following paralysis may be achieved at any point following injury with tendon transfers to replace the paralysed muscle function or nerve transfers to restore innervation to the paralysed muscle. Loss of a muscle-tendon unit through trauma may be reconstructed using tendon transfers because the new function is independent of the paralysed muscle. In longstanding paralysis beyond 12 months in lower motor neuron injury, there is irreversible collapse of the intra-muscular neural plexus and useful reanimation cannot be achieved for a complete injury beyond 12 months. Importing a new muscle as a functioning free muscle transfer with innervation through a nerve transfer may be utilised to provide function when there are no locally suitable tendon transfer options available. Tendon transfers can be undertaken at any stage as long as the principles are adhered to. They can salvage function when primary nerve reconstruction has failed to provide a useful function. Nerve transfers are time limited and can be used in isolation or as a hybrid reconstruction when combined with tendon transfers. Nerve transfers may be preferable in situ ations where tendon transfers are limited or outcome is usually limited and sub-optimal. Restoration of all functional losses is usually not possible and judicious use of arthrodesis as an adjunct may free muscle-tendon units or nerves for more important transfers to improve the functional status of the limb. Tendon transfers require more extensive dissection, immobilisation and early rehabilitation. Nerve transfers are more targeted, restoring motor function to the original muscle. Nerve transfers need less early rehabilitation, but more lengthy recovery for 12–24 months before full recovery is achieved. The function restored is more normal with nerve transfers but the decision to proceed with a nerve transfer must be made early. Typically, nerve transfers are used where there is a complete non-reconstructable nerve injury such as loss of shoulder function after C5 avulsion injury. The success of nerve transfers in partial brachial plexus injury reconstruction has resulted in exploration of their potential in other causes of paralysis and a replacement for tendon transfers or as a hybrid reconstruction in conjunction with tendon transfers for potentially greater functional gains for a particular injury. This review focuses on the principles of both tendon and nerve transfers and details the options for reconstruction of different functions following paralysis in the upper limb.
Principles of Tendon Transfer
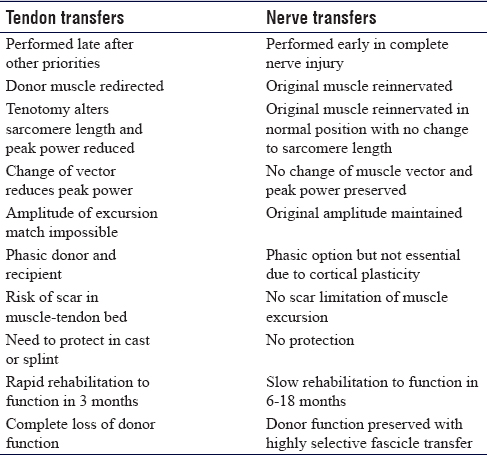
Tendon transfers are the most established technique for functional restoration of paralysis. An additional benefit of tendon transfer is that they may be used to reconstruct function where the original muscle-tendon unit is lost through tumour resection or trauma. They are robust, reliable and provide a relatively rapid solution with useful function within 6–12 weeks of surgery. Brand et al. defined the essential criteria for successful transfer and defined the importance of matching amplitude of excursion in donor–recipient muscles that are phasic.[1] Reconstruction is typically delayed until skeletal and soft-tissue conditions are optimised. As long as the majority of the Brand criteria are met successful tendon transfer can be accomplished at any stage after onset of paralysis, even after many years [Table - 1]. Following the repair of a nerve injury, sufficient time can be given to waiting for a final functional outcome before committing to tendon transfers. The techniques are well described with high-quality outcomes studies defining the anticipated outcome. Surgery requires multiple small incisions to mobilise the donor muscle, re-route the tendon for transfer and perform the tenorrhaphy.

Tenotomy of the donor and changing the vector of pull result in subtle alteration of the resting length of the sarcomere and a net loss of contraction power. Failure to restore a suitable tension can shift the mechanical force of contraction on the Blix curve,[1],[2],[3],[4] creating insufficient power for the required function. Scar in the donor muscle-tendon bed may further restrict excursion and limit the amplitude and power of contraction. Crossing several joints reduces the power of action at the desired joint. however, maintaining a chain of mobile joints allows tenodesis control of transferred tendon tension and compensating for amplitude mismatch. Tendon transfers must be protected after surgery and the rehabilitation is intense and controlled to maintain muscle-tendon glide, prevent joint stiffness and encourage relearning of the new transfer. Traditional tenorrhaphy techniques including the Pulvertaft weave are superseded by side-to-side suturing with less bulk and stronger repairs that can facilitate earlier rehabilitation and removal of protective splints.[5],[6],[7]
For patients who have lived with paralysis for a long time and adapted to their paralysis the decision to undergo surgery, temporarily lose function due to limb immobilisation and donor muscle sacrifice with uncertainty of outcome, is a challenging one. The expectation is of functional gains and the ideal preparation is to have a comprehensive pre-operative functional evaluation with a hand therapist using prioritised goals to plan and judge the outcome of surgery. The Canadian Occupational Performance Measure is suitable for this evaluation.[8]
The reality of reconstruction of paralysis following complex trauma is that there are many factors that influence the functional outcome including adequacy of a transfer for the required function, donor muscle strength, joint stiffness, compliance and number of muscles affected. Restoration of shoulder abduction remains a challenge with the trapezius transfer to the humeral head providing limited abduction excursion and strength. External rotation is more readily accomplished with transfer of the latissimus dorsi or the contralateral lower trapezius. Similarly, elbow flexion restoration may be accomplished with transfer of the triceps, pectoralis major, latissimus dorsi or augmented with a Steindler flexorplasty. All techniques restore only a part of the normal functional excursion. Elsewhere, the functional gains in the wrist and hand may be more satisfactory. Multiple tendon transfers for a high radial nerve palsy significantly improve functional use of the hand with relatively little downside. Finger extension independence and moderate loss of wrist flexion are tolerable for most patients.
Principles of Nerve Transfer
Nerve transfer is a technique that involves reanimation of a paralysed muscle using a functioning nerve near its motor point. The donor nerve may be a named motor nerve to an expendable muscle (nerve transfer) or a fascicle from within a functioning nerve between branch points (highly selective fascicle transfer). The donor nerve is sectioned distally and the recipient motor branch to the paralysed muscle is sectioned proximally and the two ends are coapted in a tension-free manner with the limb extended. The neurorrhaphy is close to the motor point and re-innervation is rapid.[9] The donor nerve should have a fascicle count ratio to the recipient nerve of at least 0.3 for a successful transfer.[10],[11]
In nerve transfer surgery, the donor loss must be inconsequential. Using the technique of highly selective transfer, there is incomplete denervation of any distal muscle due to inter-fascicular branching and redundancy within the nerve trunk of a mixed nerve. The donor muscle remains functional and any denervated muscle fibres are adopted by collateral sprouting from nearby neuromuscular junctions creating larger motor units.[12]
Following a lower motor neuron injury, there is irreversible change in the muscle and the intra-muscular neural plexus such that successful functional re-innervation in a complete nerve injury will not be possible after 12 months. Nerve transfer is, therefore, time critical and should be completed ideally by 6–9 months from injury. In a situation where there is no recovery potential in the primary nerve injury, early transfer is recommended. Tung and Mackinnon write, 'time is muscle,'[13] as after 2 years of denervation muscle fibres have disintegrated and have begun to be replaced by adipose cells. The earliest possible repair following injury is always advised as target muscle must be trophic for successful rehabilitation and 'optimal muscle re-innervation depends on sufficient quantity of regenerating motor axons reaching their target muscles within approximately 1 year after injury'.[9] The effects of chronic denervation and chronic axotomy were investigated by Holmes and Young in 1942.[12] Fu and Gordon in 1995[14] noted that the optimum results for muscle re-innervation were achieved when a freshly denervated muscle was re-innervated by an acutely axotomised nerve. This is not a typical clinical situation where there may be a delay to diagnosis, and initial attempts to repair a nerve injury and potential donor nerves may also have been injured, albeit at a lower grade and with faster recovery. The situation where a partial re-innervation from primary nerve surgery is augmented by later nerve transfer is poorly understood. There may be an opportunity to extend the window for re-innervation in such cases through first achieving giant non-functional motor units that prevent full trophic deterioration and enhanced re-innervation with a greater axon pool by salvage transfer of an intact motor nerve with abundant axons at a later date. This is controversial and unproven, but Afshari et al. have published their results using this technique in chronic degenerative spondyloradiculopathy. The paper demonstrated Medical Research Council (MRC) Scale for Muscle Strength grade 4 power recovery in three cases with spondyloradiculopathy.[15]
For practical purposes, the re-innervation should be completed within 12 months from the onset of paralysis. The situation is different in pure upper motor neuron paralysis or combined upper and lower motor neuron injury as is seen in patients with spinal cord injury. Wallerian degeneration may have been incomplete and the possibility of a successful late transfer is dependent on the findings on surgical exploration and intra-operative stimulation of the target muscle. If it still contracts, then a nerve branch from a muscle under volitional control may be transferred to it and a successful outcome still achieved even many years after the spinal cord injury on tetraplegia nerve transfers.[16],[17] Further discussion of nerve transfer reconstruction in spinal cord injury cases is beyond the scope of this review.
Nerve transfer can be completed early in a patient's recovery from injury and neural recovery may be simultaneous with other processes such as fracture healing that may preclude early tendon transfer. The limb must be rested to prevent disruption of the transfer for approximately 3 weeks, after which neural gliding and joint mobilisation therapy are commenced. The donor nerve is recruited to strengthen the stimulation in the regenerating axons and the focus in the early phase of rehabilitation is visual imagery and donor activation. The first signs of re-innervation are deep muscle tenderness which pre-dates visible contraction by approximately 6 weeks. At this stage, donor activation causes the recipient muscle to contract and this can be facilitated with surface electromyographic feedback, use of surface nerve stimulators and donor facilitation and potentiation. Central relearning continues with guided motor imagery and bimanual activation with the use of mirrors. The later phase of rehabilitation involves central re-mapping with selective activation of the recipient without donor recruitment and facilitation. This plasticity phase takes between 12 and 24 months for most upper limb nerve transfers.
Nerve transfer may be employed in isolation or in combination with tendon transfers for complex paralysis cases. In the upper limb tendon transfer, restoration of shoulder abduction and elbow flexion is poor and nerve transfer, developed in C5 and C6 brachial plexus injuries, produces excellent functional results from re-innervation of the original muscle left paralysed by injury. For some functions, nerve transfers are superior to tendon transfers. Nerve transfers for complex muscles or nerves with multiple functions such as the deltoid, digital flexors, digital extensors and motor ulnar nerve for the hand intrinsic muscles may provide more independent function than a traditional tendon transfer where the whole function is replaced with one muscle-tendon transfer. Highly selective fascicle transfer may allow additional gains over tendon transfer alone without complete denervation of the donor muscle. In tetraplegia, upper limb reconstruction creates the potential for an overall higher level of function than would be achievable with traditional tendon transfers alone. As for tendon transfers, selected joint arthrodesis in complex paralysis cases may free nerves for transfer to more useful functions at the same time as providing skeletal stability and reducing the total number of functions requiring reconstruction [Table - 2].

Comparison
There are advantages and disadvantages to both tendon transfer and nerve transfer in the reconstruction of paralysis. Tendon transfers typically require skeletal stability and tissue homeostasis with an engaged patient who has the cognitive ability to understand and undertake rehabilitation. If the above is not adhered to, there is a risk of scar tissue forming around the muscle-tendon transfer and limiting functional movement. Nerve transfers can be completed early without the need for rehabilitation of the transfer until muscle re-innervation has occurred. Typically, however, in cases where a nerve injury is repaired, there is a need to wait to establish if recovery is inadequate before proceeding with late tendon transfers as a salvage option. This creates a challenge for the timing of a nerve transfer. Generally, nerve transfers must be performed as early as possible after the onset of paralysis to be successful and decision-making is simple in non-reconstructable nerve lesions, like nerve root avulsions. It is more difficult in cases where reconstruction of a nerve gap with grafts may result in some functional recovery, but final assessment cannot be made until 12-18 months later at which point it is beyond the window for successful nerve transfer. The results of nerve transfer in complex nerve injury cases are so good and predictable that specialist peripheral nerve surgeons will use nerve transfer early in cases of high-grade proximal peripheral nerve injury and poor surgical beds for grafting and where there is diagnosis and referral delay such that primary nerve gap reconstruction alone would be likely to fail. Combinations of nerve and tendon transfers generally achieve the best results in complex cases. Tendon transfers may be used for late salvage of a failed nerve transfer although this is an uncommon situation and probably related to inappropriate timing of the technique, poor donor nerve quality, poor patient selection or a technical issue such as tension or disruption at the coaptation site [Table - 3].
Creating a Functional Upper Limb
Due to variations in injury patterns and severity, there is no strategy that can be selected for every functional reconstruction. Nerve transfer involves complex assessment and decision-making and careful microsurgical intra-neural dissection and as such is the remit of a specialised peripheral nerve surgeon. Tendon transfers produce more reliable results in less experienced hands; however, the best results of tendon transfers are achieved by those undertaking these types of reconstruction regularly as there are many technical points regarding muscle mobilisation, routing and tensioning that influence the outcome. The aims of reconstruction should be to restore useful function without creating further deficits, in a time frame that is acceptable to the individual patient. A systematic examination noting the MRC grade of key muscles, evaluating joint mobility and soft tissue envelopes is essential before planning reconstruction. The patient must be involved in the decision-making process, and a thorough explanation of the rehabilitation pathway, anticipated outcome and potential complications must be discussed before surgery. Early involvement of a hand therapist and an upper limb physiotherapist can prepare the patient for surgery. While there are some classic patterns of paralysis and reconstructive options such as a double nerve transfer for C5 avulsion (CN-XI to supra-scapular nerve and medial triceps to axillary nerve), a quadruple transfer for C5–6 avulsion or upper trunk rupture (CN-XI to supra-scapular nerve, medial triceps to axillary nerve, ulnar nerve fascicle to brachialis and median nerve fascicle to biceps) and tendon transfers for high radial palsy (pronator teres to extensor carpi radialis brevis, flexor carpi radialis to extensor digitorum and palmaris longus to extensor pollicis longus), there are numerous options available and complex patterns of paralysis that require a bespoke reconstruction solution. [Table - 4] details common tendon and nerve transfer options for critical functions. It is not comprehensive and certain tendon or nerve transfer options may not be available in individual cases.
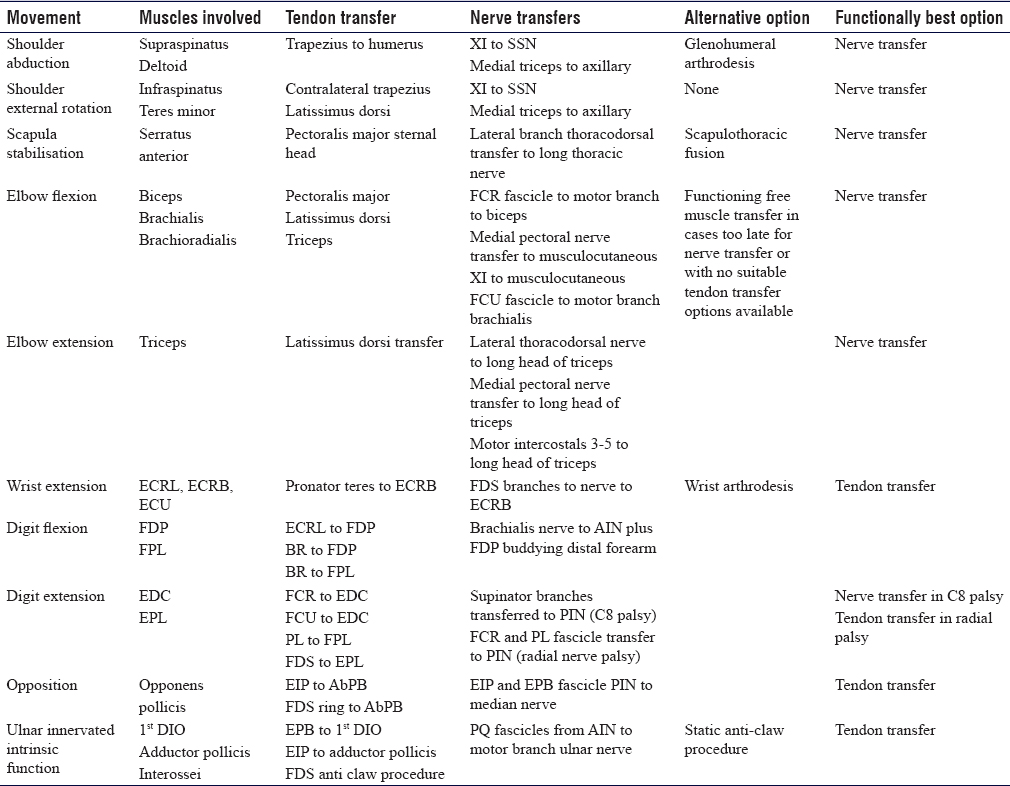
While these procedures are often performed as separate entities, it is important to note that they should be added to the 'surgical tool box' to be considered as independent procedures, as a second line should one fail or used as a 'hybrid.' These hybrid procedures combine the benefits of each to create the most functional outcome, for example, a Steindler flexorplasty (advancement of the flexor/pronator origin)[17] and Oberlin transfer for elbow flexion.[18] High radial nerve injuries can be treated with a pronator teres tendon transfer to the wrist to correct wrist drop and a median nerve fascicle to flexor carpi ulnaris to the posterior interosseous nerve to allow for finger and thumb extension.[19] However, potential donor morbidity should be considered before undertaking any tendon, nerve or hybrid transfer for the reconstruction of paralysis.
Conclusion
Surgeons involved in the reconstruction of function after paralysis should understand the underlying cause, exclude the option of nerve decompression or reconstruction and understand the functional hierarchy of the upper limb. Potential functional loss from donor muscle-tendon or nerve harvest should be considered as well as the rehabilitation requirements. The optimum strategy for an individual patient must be selected based on a number of criteria including the time anticipated for functional restoration. Muscle-tendon transfer, nerve transfer or hybrid techniques involving both procedures may be considered. There are well-proven strategies for common conditions with long-term outcome data available for tendon transfers. Nerve transfer surgery is established in the management of brachial plexus injuries, and the success in this field has led to the application of the technique to peripheral nerve injury, but the evidence base is limited with no good comparative studies published to date. Complex paralysis requires a bespoke solution. The integration of a hybrid approach in such cases increases the reconstruction possibilities and potentially greater functional gains than for muscle-tendon transfer or nerve transfer techniques used in isolation.
Ethical consideration
The article is a review based on current clinical practice of the senior author. The paper did not require ethics board review.
Acknowledgement
With thanks to the Peripheral Nerve Injury Service, University Hospitals Birmingham.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Author's contribution
DCC Prepared draft and final manuscript. PM Reviewed manuscript and provided logistical support. DMP Conceived the concept for the paper, edited the paper and approved the final draft. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Brand PW, Beach RB, Thompson DE. Relative tension and potential excursion of muscles in the forearm and hand. J Hand Surg Am 1981;6:209-19. [Google Scholar] |
| 2. | Fridén J, Lieber RL. Evidence for muscle attachment at relatively long lengths in tendon transfer surgery. J Hand Surg Am 1998;23:105-10. [Google Scholar] |
| 3. | Fridén J, Pontén E, Lieber RL. Effect of muscle tension during tendon transfer on sarcomerogenesis in a rabbit model. J Hand Surg Am 2000;25:138-43. [Google Scholar] |
| 4. | Lieber RL, Pontén E, Burkholder TJ, Fridén J. Sarcomere length changes after flexor carpi ulnaris to extensor digitorum communis tendon transfer. J Hand Surg Am 1996;21:612-8. [Google Scholar] |
| 5. | Fridén J, Tirrell TF, Bhola S, Lieber RL. The mechanical strength of side-to-side tendon repair with mismatched tendon size and shape. J Hand Surg Eur Vol 2015;40:239-45. [Google Scholar] |
| 6. | Tsiampa VA, Ignatiadis I, Papalois A, Givissis P, Christodoulou A, Fridén J, et al. Structural and mechanical integrity of tendon-to-tendon attachments used in upper limb tendon transfer surgery. J Plast Surg Hand Surg 2012;46:262-6. [Google Scholar] |
| 7. | Fridén J, Lieber RL. Tendon transfer surgery: Clinical implications of experimental studies. Clin Orthop Relat Res 2002;403:S163-70. [Google Scholar] |
| 8. | Law M, Baptiste S, McColl M, Opzoomer A, Polatajko H, Pollock N, et al. The canadian occupational performance measure: An outcome measure for occupational therapy. Can J Occup Ther 1990;57:82-7. [Google Scholar] |
| 9. | Brunelli G, Brunelli F. Partial selective denervation in spastic palsies (hyponeurotization). Microsurgery 1983;4:221-4. [Google Scholar] |
| 10. | Tötösy de Zepetnek JE, Zung HV, Erdebil S, Gordon T. Innervation ratio is an important determinant of force in normal and reinnervated rat tibialis anterior muscles. J Neurophysiol 1992;67:1385-403. [Google Scholar] |
| 11. | Schreiber JJ, Byun DJ, Khair MM, Rosenblatt L, Lee SK, Wolfe SW, et al. Optimal axon counts for brachial plexus nerve transfers to restore elbow flexion. Plast Reconstr Surg 2015;135:135e-41e. [Google Scholar] |
| 12. | Holmes W, Young JZ. Nerve regeneration after immediate and delayed suture. J Anat 1942;77:63-96. [Google Scholar] |
| 13. | Tung TH, Mackinnon SE. Nerve transfers: Indications, techniques, and outcomes. J Hand Surg Am 2010;35:332-41. [Google Scholar] |
| 14. | Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged denervation. J Neurosci 1995;15:3886-95. [Google Scholar] |
| 15. | Afshari FT, Hossain T, Miller C, Power DM. Salvage of cervical motor radiculopathy using peripheral nerve transfer reconstruction. Br J Neurosurg 2018;10:1-5. [Google Scholar] |
| 16. | Fox IK, Davidge KM, Novak CB, Hoben G, Kahn LC, Juknis N, et al. Use of peripheral nerve transfers in tetraplegia: Evaluation of feasibility and morbidity. Hand (N Y) 2015;10:60-7. [Google Scholar] |
| 17. | Steindler A. A muscle plasty for the relief of flail elbow in infantile paralysis. Interstate Med J 1918;2:235-41. [Google Scholar] |
| 18. | Teboul F, Kakkar R, Ameur N, Beaulieu JY, Oberlin C. Transfer of fascicles from the ulnar nerve to the nerve to the biceps in the treatment of upper brachial plexus palsy. J Bone Joint Surg Am 2004;86-A: 1485-90. [Google Scholar] |
| 19. | Power D. Hybrid Tendon Transfer and Nerve Transfer Reconstruction of High Radial Nerve Palsy Surgical Technique. Orthoracle. Available from: https://www.orthoracle.com/library/hybrid-tendon-transfer-and-nerve-transfer-reconstruction-of-high-radial-nerve-palsy/. [Last accessed on 2018 Dec 12]. [Google Scholar] |
Fulltext Views
6,376
PDF downloads
2,263





