Translate this page into:
A novel technique of using percutaneous nerve catheter for post-operative analgesia and early mobilization in hand surgeries
*Corresponding author: Megha Thaleppady, Assistant Professor, Department of Anesthesiology, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India megha.t@manipal.edu
-
Received: ,
Accepted: ,
How to cite this article: Kamath BN, Nayak KR, Thaleppady M, Kamath KR. A novel technique of using percutaneous nerve catheter for post-operative analgesia and early mobilization in hand surgeries. J Musculoskelet Surg Res. 2024;8:41-6. doi: 10.25259/JMSR_180_2023
Abstract
Objectives:
The need for the early mobilization of the fingers after hand surgery cannot be overemphasized in the outcome of the patients. In hand surgeries, the open method of blocking the distal nerves at the distal forearm is well-known and practiced in some centers. The same with a closed method has not been practiced or published in the English literature.
Methods:
We performed the technique soon after the surgery when the patient was still under regional anesthesia/general anesthesia. Pain score was assessed using a numeric pain rating scale (NPRS) between post-operative day 1 and day 4 during the finger mobilization. Active metacarpophalangeal (MCP), proximal interphalangeal (PIP), and distal interphalangeal (DIP) joint movements were recorded using a goniometer.
Results:
NPRS improved from 2.88 ± 0.81 at post-operative day 1–1.69 ± 0.48 at post-operative day 4. Similarly, the range of motion of the MCP joint improved from 66.67 ± 12.31° to 82.67 ± 8.877°. The range of motion of the PIP joint significantly improved from 69.38 ± 26.95° to 85 ± 11.55°, respectively. Similarly, the range of motion of the DIP joint improved from 85 ± 11.55° to 69 ± 11.55°. We have not encountered any major complications such as infection, hematoma, or injury to tendons or nerves.
Conclusion:
The percutaneous nerve catheter is an excellent technique in hand surgeries for blocking peripheral nerves, thus providing analgesia and early rehabilitation.
Keywords
Closed nerve block
Early mobilization
Fingers
Infant feeding tube
Nerve block catheter
Pain relief
Post-operative
INTRODUCTION
Effective anesthesia is of paramount importance for any wrist or hand surgery. Postoperatively, 50–70% of patients experience moderate to severe pain.[1] This will hamper the post-operative results as some surgeries need the early mobilization of the fingers. The current trend in hand surgery, where patients are compelled to take part in early rehabilitation programs involving active or passive finger mobilization, shows favorable results in cases such as stable osteosynthesis of phalangeal and metacarpal fractures, carpal tunnel release, tenolysis, arthrolysis, wrist arthroscopy, and tendon repair.[2]
For many of the aforementioned surgeries, peripheral nerve blocks are a quick, easy, and highly effective technique with no systemic side effects. Although there are few contraindications to such peripheral blocks, local infection at the needle insertion site and an allergy to local anesthetic are the most frequently mentioned.[3] It is well known that the continuous peripheral catheter technique is used to block the brachial plexus.[1] However, it cannot be used in postoperative mobilization since it blocks both the sensory and motor distributions, paralyzing the fingers’ long flexors and extensors.[4] The method of using a percutaneous catheter to block the peripheral nerves at the wrist level to provide anesthesia and analgesia continuously has not been studied in the literature so far. We describe a technique of blocking nerves primarily for the early fingers mobilization.
MATERIALS AND METHODS
We carried out a prospective study that included adult patients who presented with various hand injuries. The technique was performed on patients only after attaining permission from the Institutional Ethical Committee clearance. We performed the technique soon after the surgery when the patient was still under regional anesthesia/general anesthesia. This procedure was performed on 16 patients between 2021 and 2022 by an experienced senior hand surgeon and anesthetist.
Pain score was assessed using a numeric pain rating scale (NPRS) between day 1 and day 4 during the mobilization of fingers. Active metacarpophalangeal (MCP) joint, proximal interphalangeal (PIP), and distal interphalangeal (DIP) joint movements were recorded using a goniometer. Range from the degree of immobilization to maximum flexion possible was recorded between post-operative day 1–4. The nerve catheter was removed on post-operative day 5 without any complications. All patients were prescribed minimal to no additional intravenous or oral analgesia.
The NPRS scale and range of motion among the joints were compared between day 1 and day 4 for differences in improvements. Differences were considered statistically significant at P < 0.05. Mean values are presented as mean ± standard deviation.
Technique
This method involves using a trocar (blunt 2 mm K-wire), cannula (3 inches 10-gauge blunted needle), and a small-sized 6-gauge infant feeding tube.
The three tendons from medial to lateral, namely, flexor carpi ulnaris (FCU), palmaris longus (PL), and flexor carpi radialis (FCR) over the distal forearm are marked [Figure 1a]. The median nerve gives a palmar cutaneous branch (PCBMN) around 5 cm proximal to the wrist crease.[5] The ulnar nerve gives a dorsal cutaneous branch (DCUBN) 5 cm proximal to the wrist crease.[6] These branches are given deep to deep fascia. The tip of the infant feeding tube should lie at this level. PCBMN initially lies between FCR and PL, and as it goes distally, it is closer to FCR. DCUBUN initially lies deep to the lateral border of FCU. These points where the nerves supplying the palm and the dorsum of the hand dorsally are marked as distal marking points [Figure 1a]. One more proximal point is marked in the middle forearm 3 inches proximal to and in line with the first marking corresponding to the origin of PCBMN and DCUBUN. The surgeon must be very conversant with this surgical and surface anatomy.

- (a) Kaplan’s lesion following open reduction. (b and c) Equipment required- a 12’ long, 2 mm K-wire with blunt ends and 10-gauge 3’ long needle, (d) 6-gauge infant feeding tube with the outer diameter 2 mm easily passing through the 10-gauge needle.
The surgeon must also have the following equipment. K-wire preferably 12 inches long with a blunt tip (specifically made blunt for this purpose); 10-gauge needle 3 inches long [Figure 1b and c]; 6-gauge infant feeding tube with an outer diameter of 2 mm [Figure 1d].
A small half-cm incision is made in the proximal marking, cutting the skin and deep fascia [Figure 2a]. A 2 mm K-wire with a blunt tip is introduced through a rent in the deep fascia [Figure 2b]. K-wire is slowly negotiated distally until its tip reaches the distal point. The needle mentioned above is taken and fed into the other end of the K-wire. The needle is then pushed slowly into the distal marking [Figure 2c]. The needle’s internal diameter (2.692 mm) is big enough to accept 2 mm K-wire. Once the tip has almost reached the distal marking point, the K-wire is then removed very slowly. The infant feeding tube is then fed into the needle and passed until it reaches the marked distal point [Figure 2d].
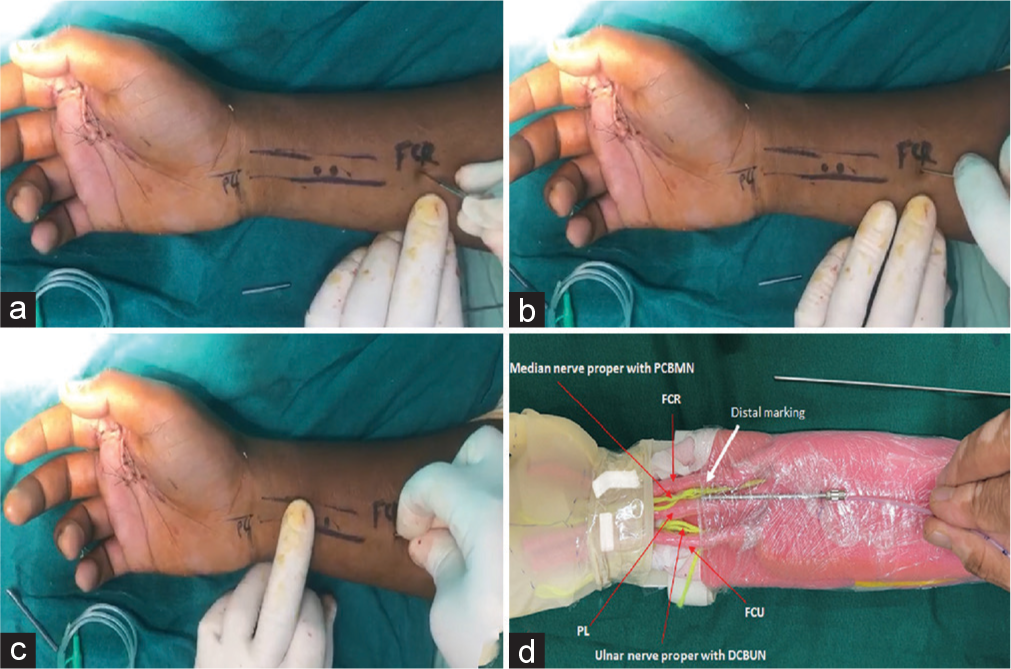
- (a) After wound closure, the distal three tendons are marked. Incision at the proximal marking point. (b) Passage of blunt 2 mm K-wire. (c) The needle passed through the incision at the proximal marking point. (d) Depiction is shown using a hand and forearm model.
The infant feeding tube is fixed in that position to the skin by a single stitch. Now, our method is ready to block the median and ulnar nerve [Figure 3a-f], including PCBMN and DCUBN, ensuring nearly the whole hand, including the integuments. The same procedure is repeated in the superficial branch of the radial nerve (SBRN) if the need arises. This leaves the extrinsic flexors and extensors to act unparalyzed. This system provides enough analgesia postoperatively for the early movement of fingers, which is paramount for a surgeon, especially in the case of tenolysis. The SBRN blocks the dorsal two-and-a-half portions of the hand proximal to the PIP crease.[7] The surgeon prefers to block eight hourly with a combination of long- and short-acting local anesthetic agents (Lignocaine and Bupivacaine).[8] Care must be taken not to inject more than 5 mL each time per catheter/nerve. The authors have maintained the catheters for 3–5 days postoperatively [Figure 4a-c].
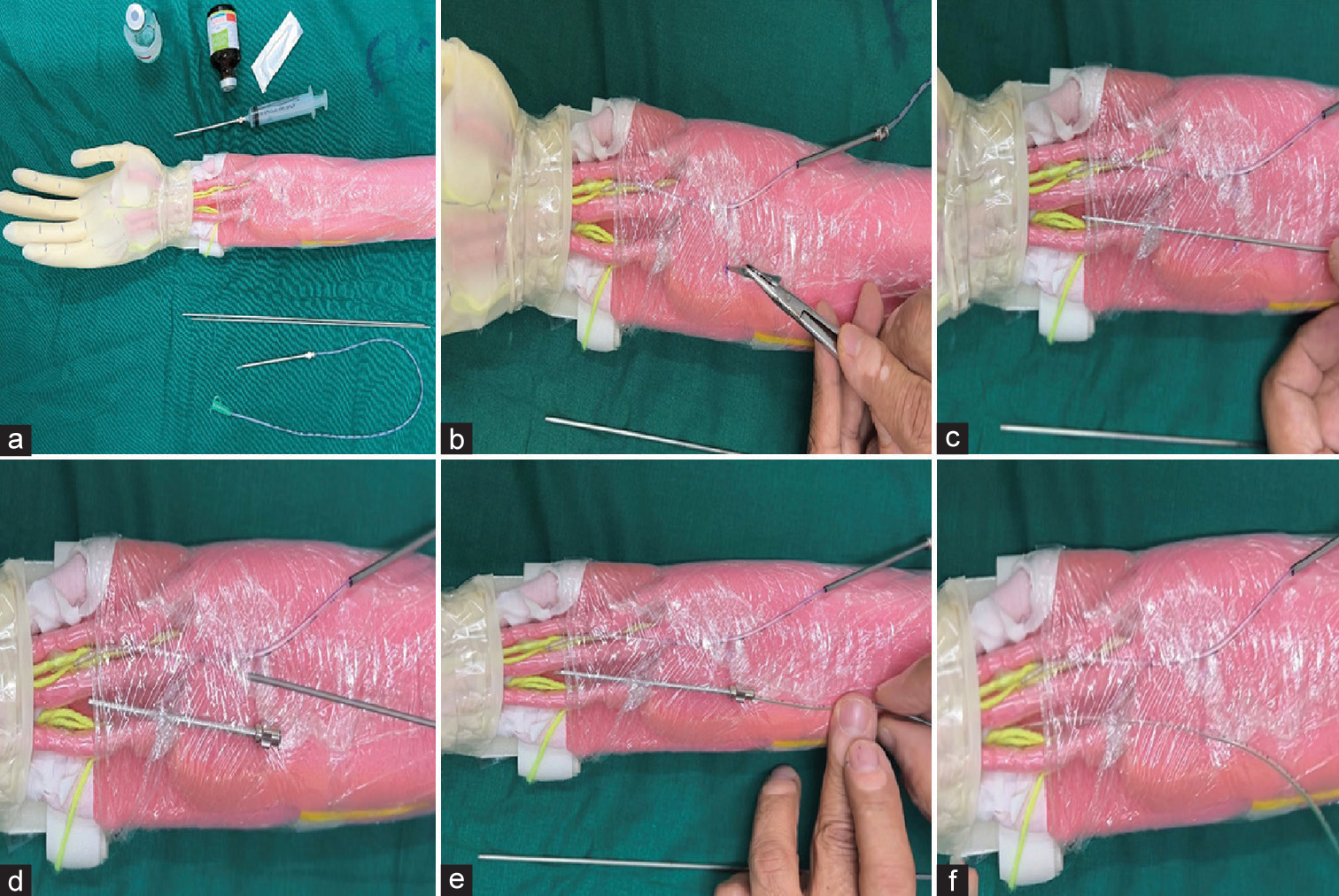
- (a) Pictorial depiction of blocking ulnar nerve using a model with the required equipment. (b) Incision made at the proximal marking point. (c) Negotiation of K-wire under the deep fascia. (d) Needle in situ after removing the wire. (e) Insertion of the infant feeding tube. (f) Showing infant feeding tube is in the appropriate place.
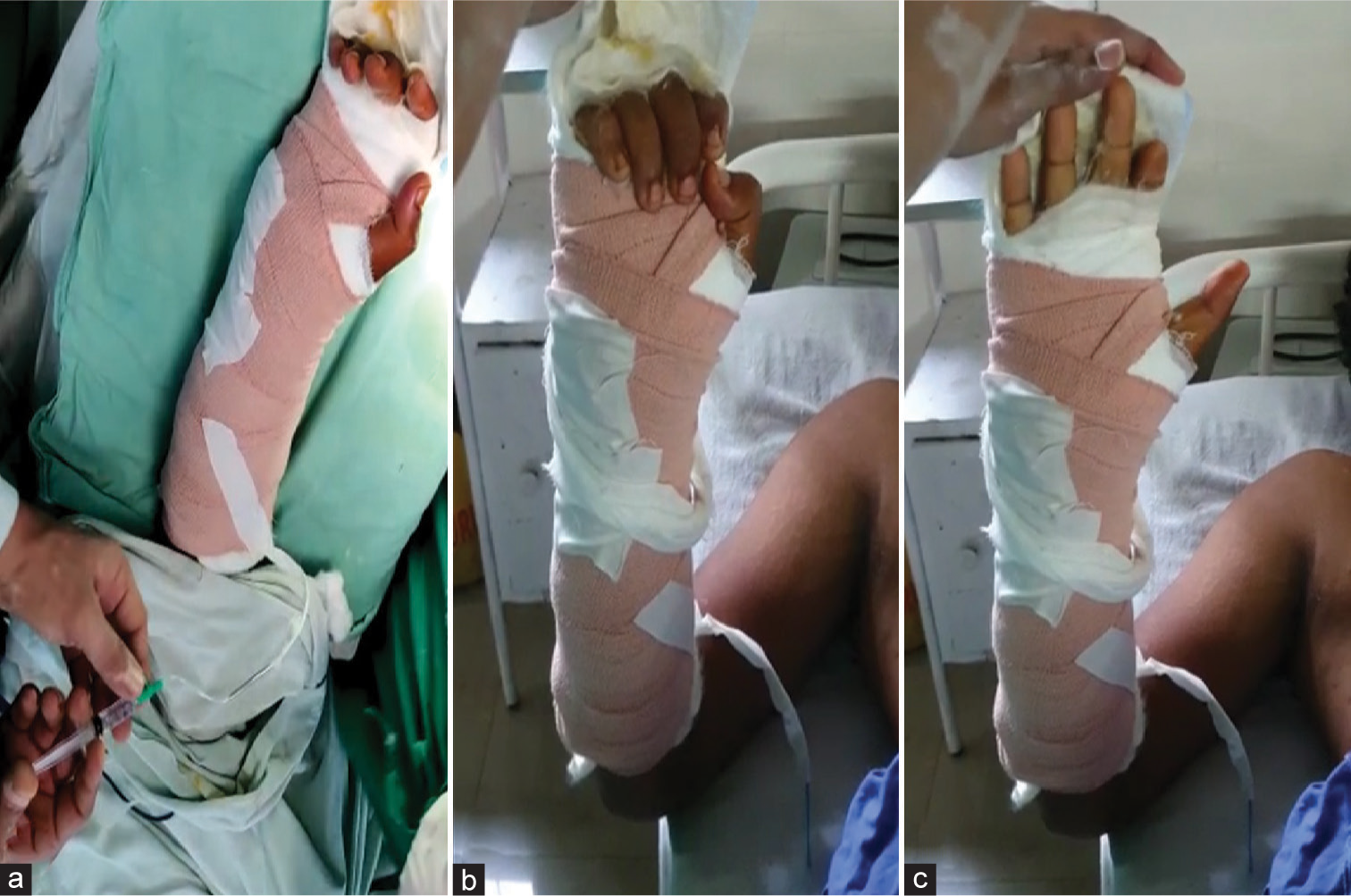
- (a) Injection of a combination of long- and short-acting anesthetics through the infant feeding tube, (b and c) showing a pain-free movement of flexion and extension of the index finger.
RESULTS
This technique was performed on 16 patients who underwent hand surgeries for various indications [Table 1]. The mean age of the participants was 33.5 ± 9.3 years and the majority were female. Thirteen patients underwent median nerve blockade, whereas the other three underwent combined median and ulnar nerve blockade.
| Case | Age | Sex | Disease | Surgery | Follow-up |
|---|---|---|---|---|---|
| 1 | 33 | M | Second proximal phalanx fracture | ORIF with plating | 1 year |
| 2 | 28 | M | Third metacarpal fracture | ORIF with plating | 1 year |
| 3 | 40 | M | Zone II flexor tendon injury of index finger | Tendon repair | 8 months |
| 4 | 28 | M | PIP joint contracture of the middle finger | Arthrolysis | 9 months |
| 5 | 43 | M | Tenosynovitis of the index and middle finger | Tenolysis | 3 months |
| 6 | 21 | M | Kaplan’s lesion of the second MCP joint | Open reduction | 6 months |
| 7 | 19 | M | Tenosynovitis of index, middle and ring finger | Tenolysis | 3 months |
| 8 | 27 | M | Kaplan’s lesion of the second MCP joint | Open reduction | 6 months |
| 9 | 36 | M | Kaplan’s lesion of the second MCP joint | Open reduction | 6 months |
| 10 | 45 | M | Zone II flexor tendon injury of index finger | Tendon repair | 6 months |
| 11 | 54 | F | PIP joint of second digit post-burn contracture | Contracture release | 1 year |
| 12 | 33 | F | Second proximal phalanx fracture | ORIF with plating | 9 months |
| 13 | 24 | M | Dupytren’s contracture of ring finger | Partial fasciectomy | 6 months |
| 14 | 32 | M | Third metacarpal fracture | ORIF with plating | 1 year |
| 15 | 29 | M | Second metacarpal fracture | ORIF with plating | 1 year |
| 16 | 44 | M | Dupytren’s contracture of ring finger | Partial fasciectomy | 8 months |
MCP: Metacarpophalangeal, ORIF: Open reduction and internal fixation
The numerical pain rating score improved from 2.88 ± 0.81 on post-operative day 1–1.69 ± 0.48 on post-operative day 4, which is highly significant (P < 0.001). Similarly, the range of motion of the MCP joint improved from 66.67 ± 12.31° to 82.67 ± 8.877°, which was highly significant. The range of motion of the PIP joint significantly improved from 69.38 ± 26.95° to 85 ± 11.55°, respectively. Similarly, the range of motion of the DIP joint improved from 60.38 ± 11.24° to 69 ± 11.55°, which was highly significant [Table 2].
| Day 1 (Mean±SD) | Day 2 (Mean±SD) | Day 3 (Mean±SD) | Day 4 (Mean±SD) | P-value | |
|---|---|---|---|---|---|
| NPRS | 2.88±0.81 | 2.25±0.68 | 1.63±0.5 | 1.69±0.48 | <0.001 |
| Range of motion of MCP joint | 66.67±12.31 | 82±7.79 | 77.33±9.97 | 82.67±8.877 | <0.001 |
| Range of motion of PIP joint | 69.38±26.95 | 75±20 | 81.88±12.23 | 85±11.55 | 0.007 |
| Range of motion of DIP joint | 60.38±11.24 | 65±11.55 | 65±11.55 | 69±11.55 | <0.001 |
NPRS: Numerical pain rating scale, MCP: Metacarpophalangeal, PIP: Proximal interphalangeal, DIP: Distal interphalangeal, SD: Standard deviation
We have not encountered any major complications such as infection, hematoma, or injury to tendons or nerves. The authors have not encountered any case where they have failed to block the concerned nerve meant for early mobilization.
DISCUSSION
To avoid adhesions and scarring, it is crucial to mobilize the fingers as soon as possible after surgery actively.[9] Due to excruciating pain after surgery, patients tend to avoid moving their fingers.[10] Therefore, administering analgesia would aid patients in active mobilization.[11] Even so, intravenous analgesics frequently have negative side effects and do not fully aid mobilization. The authors think that a continuous peripheral sensory block provides excellent analgesia while also being a great option for finger rehabilitation.
Watanabe et al.[12] in their study, the mean visual analog scale was 4–5 during the rehabilitation, whereas in Otsuka et al.,[13] the study showed a mean score of 1–2. We also found similar results, which even improved on post-operative day 4. All the patients had excellent analgesia, which did not restrict finger mobilization.
In their study, Otsuka et al.[13] compared the range of motion of joints in hands preoperatively and postoperatively for flexion and extension and found excellent results. We compared the range of motion of small joints between post-operative day 1 and day 4 and showed significant improvement. Since we had mixed cases, the pre-operative range of motion of joints was not included. Our study found that continuous peripheral percutaneous catheter improved finger rehabilitation significantly.
Otsuka et al.,[13] in their study, used a 2 cm incision over the distal forearm for the catheter insertion. Our study used a percutaneous technique with no need for post-procedural sutures.
Although we do not recommend ultrasonography (USG) in every case, we have demonstrated (by doing a USG) if the tip of the infant feeding tube will lie close to the median/ulnar nerves/PCBMN/DCBUN so that the block is complete involving the various branches of these nerves blocking almost the entire hand [Figure 5]. In addition to the early mobilization, this will aid in pain management, requiring fewer doses of parenteral analgesia.[14]

- The tip of the infant feeding tube placed near the median nerve.
The disadvantage of this method is that the blunt needle cannot be removed and will be there with the patient as long as the infant feeding tube is in place. This may interfere with the post-operative radiographs of the forearm if needed. Hence, it is imperative that these needles are placed and stuck to the slab in such a way that it interferes with the postoperative radiographs in the least possible ways.
To the best of the authors’ knowledge, such a closed method of blocking the distal nerves in the upper limb for early mobilization has not been described in any English literature. This novel minimally invasive technique will take us one step further in the early rehabilitation of patients undergoing various hand surgeries.
CONCLUSION
We describe a simple, easy, and reproducible technique to meet the surgeon’s need for analgesia and early post-operative mobilization of fingers with the help of unparalyzed extrinsic muscles of the fingers. Thorough knowledge of surface and surgical anatomy is very important. The equipment needed is readily available in any operation theatre.
AUTHOR’S CONTRIBUTIONS
BJK conceived and designed the study, conducted research, and provided research materials. MT organized and collected data. KRN analyzed and interpreted data. KRK wrote the initial and final draft of the article and provided logistic support. All authors have critically reviewed and approved the final draft and are responsible for the manuscript’s content and similarity index.
ETHICAL APPROVAL
The study was conducted after attaining clearance from the Institutional Ethical Clearance. IEC KMC MLR 12-2020/425 KMC Mangalore. (December 24, 2020)
DECLARATION OF PATIENT CONSENT
The authors certify that they have obtained all appropriate patients consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY FOR MANUSCRIPT PREPARATION
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
CONFLICTS OF INTEREST
There are no conflicting relationships or activities.
FINANCIAL SUPPORT AND SPONSORSHIP
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- The role of continuous peripheral nerve blocks. Anesthesiol Res Pract. 2012;2012:560879.
- [CrossRef] [PubMed] [Google Scholar]
- Mobilization précoce passive en chirurgie de la [Early passive mobilization in surgery of the hand] Ann Chir Main. 1989;8:356-61.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-articular and portal infiltration versus wrist block for analgesia after arthroscopy of the wrist: A prospective RCT. Bone Joint J. 2015;97B:1250-6.
- [CrossRef] [PubMed] [Google Scholar]
- Can we perform distal nerve block instead of brachial plexus nerve block under ultrasound guidance for hand surgery? Eurasian J Med. 2016;48:167-71.
- [CrossRef] [PubMed] [Google Scholar]
- Is palmar cutaneous branch of the median nerve more swollen in carpal tunnel syndrome? Ann Rehabil Med. 2021;45:325-30.
- [CrossRef] [PubMed] [Google Scholar]
- Anatomy, shoulder and upper limb, ulnar nerve In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
- [Google Scholar]
- Applied anatomy of the superficial branch of the radial nerve. Clin Anat. 2008;21:38-45.
- [CrossRef] [PubMed] [Google Scholar]
- Buffered lidocaine and bupivacaine mixture-the ideal local anesthetic solution? Plast Surg (Oakv). 2015;23:87-90.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of early active mobilization after flexor tendons repair in zones II-V in hand. Indian J Orthop. 2010;44:314-21.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of unfavourable results of flexor tendon surgery: Ruptured repairs, tethered repairs and pulley incompetence. Indian J Plast Surg. 2013;46:458-71.
- [CrossRef] [PubMed] [Google Scholar]
- Management of pain induced by exercise and mobilization during physical therapy programs: Views of patients and care providers. BMC Musculoskelet Disord. 2011;12:172.
- [CrossRef] [PubMed] [Google Scholar]
- Alternative site for median nerve blockade allowing early functional rehabilitation after hand surgery. Can J Anaesth. 2012;59:58-62.
- [CrossRef] [PubMed] [Google Scholar]
- Continuous peripheral nerve blocks for early active mobilization after hand surgery: Four case reports. J Hand Surg Asian Pac Vol. 2018;23:419-23.
- [CrossRef] [PubMed] [Google Scholar]
- Motor-sparing continuous median nerve block for hand surgery: A pediatric case. Asian J Anesthesiol. 2017;55:78-9.
- [CrossRef] [PubMed] [Google Scholar]






