Translate this page into:
Adolf stoffel and the development of peripheral neurosurgical reconstruction for the management of paralysis: One hundred years of nerve transfer surgery
Corresponding Author:
Mohammad Nassimizadeh
The Peripheral Nerve Injury Service, Queen Elizabeth Hospital, Birmingham
UK
m.nassimizadeh@gmail.com
| How to cite this article: Nassimizadeh M, Nassimizadeh AK, Power DM. Adolf stoffel and the development of peripheral neurosurgical reconstruction for the management of paralysis: One hundred years of nerve transfer surgery. J Musculoskelet Surg Res 2019;3:40-46 |
Abstract
The concept of nerve transfer as an alternative solution for paralysis through rewiring a denervated motor nerve using an intact and expendable nerve branch or fascicle in proximity to the injured nerve became widely adopted as a strategy for nerve root avulsion in brachial plexus injuries. The success of the technique has encouraged clinicians to extend the indications for other complex nerve injuries, and the technique may be considered as an adjunct or replacement to nerve grafting for some proximal, high-energy, and late-presenting peripheral nerve injuries. The technique is not new. Many of the current “advances” in peripheral nerve transfer were described contemporaneously by Adolf Stoffel in 1911. He was an orthopedic surgeon working in Germany in the early 1900s. He had an interest in nerve injury reconstruction, described the functional fascicular anatomy of the peripheral nerves, developed an intraoperative nerve stimulator, and pioneered many of the techniques used today. His work was never translated from the German language and did not, therefore, receive wide acknowledgment. The aim of this study is to correct the perceived timeline of nerve transfer surgery and include the pioneering work of Adolf Stoffel.
Introduction
Peripheral nerves were first described in 300 BC when Herophilus distinguished them from tendons by dissecting them to the spinal cord.[1] Rhazes then made the first reference to nerve repair in 900 AD. Initial attempts at nerve repair were first noted by medieval surgeons.[2],[3] But it was in 1795 that Cruikshank demonstrated recovery of the distal nerve and functional recovery following repair.[4],[5] Subsequently, Muller studied rabbit sciatic nerve and further advanced the understanding of nerve repair physiology.[6] Once this basis was established, various surgical innovations followed with numerous techniques were described, including side-to-side repair[7] and epineural flap repair.[8] In the 1850s, Waller described light microscopy changes in the injured nerve.[9],[10] This was followed in 1864 by Nelaton who described secondary nerve repair and by Hueter in 1873 who described primary epineural nerve suturing.[11] Refinements followed with Mikuliez who described sutures in 1882 and Loebke who described bone shortening to reduce repair tension in 1884.[12] Nerve grafting was described in 1876 by Albert.[12]
Then, in the early 1900s, Cajal described axonal regeneration from the proximal nerve stump after transection injury. In 1906, Sherren demonstrated xenografting of nerves,[13] and in 1917, median nerve allografting was reported by Mayo-Robson.[14]
Advances in the understanding of nerve injury and recovery followed major conflicts with many young men sustaining complex limb injuries, pain, and paralysis following nerve injury. In his book “Injuries of Nerves,” Mitchell reported his experience managing patients with nerve injuries sustained during the American Civil War, and the complex pain injury patterns of main mixed motor-sensory nerve trunk injury were defined.[15] In Europe at the same time, Foerster was refining the techniques for nerve repair that lead to advances in management during the Great War.[16],[17],[18] In 1911, Tinel in France and Hoffman in Germany both described lightly percussing over an injured nerve to elicit a sensation of pins and needles or tingling in the regenerating nerves' distribution.[19]
Seddon, during the Second World War, documented the variable results of primary nerve repair, secondary nerve repair, and nerve grafting.[20] He later published a classification of nerve injury dependent on the continuity and potential for functional recovery of the injured nerve.[21] He defined the terms neurapraxia, axonotmesis, and neurotmesis.
Sunderland described the architecture of peripheral nerves[22] with a classification system to build on that of Seddon that explained the variable outcomes from axonotmesis injuries.
The development of fine suture materials, specialist instrumentation, and the operating microscope in the 1960s enabled accurate fascicular repair and the foundation for nerve grafting of gaps in peripheral nerves to be more widely adopted.
Nerve Structure and Function
The epineurium is the outermost, loose, connective tissue layer that surrounds groups of fascicles, protecting against external trauma. It is thickest near joints to protect against compression and friction.[23] Fascicle groups are bound together by interfascicular epineurium. Each fascicle contains axons in endoneurial tubes bound together by perineurium. The perineurium is strong and maintains neural mechanical integrity.[24] Endoneurium is a collagenous tissue layer, which surrounds individual axons. It resists longitudinal strain and supports the formation of the endoneurial tube. Blood vessels lie in the epineurium with transverse perforating vessels supplying oxygen and nutrients to the fascicles. Neurons are the electrically excitable cells with its axon projections designed for transmitting action potentials and forming connections with other neurons or end organs. Schwann cells form the basic cellular support components of the peripheral nervous system. They provide support to the axons with specialized cells forming myelinated coverings for large, fast-conducting axons. Motor axons have a cell body located in the anterior horn of the spinal cord. Sensory axons have a cell body in the dorsal root ganglion, outside the spinal cord.
A motor unit is a group of muscle fibers innervated by one axon.[25] The axon has specialized terminals that release neurotransmitters into a cleft, and binding to receptors on the muscle fibers initiates muscle cell depolarization, calcium release from the sarcoplasmic reticulum, and contraction through activation of the actin-myosin system.
Nerve Injury
Wallerian degeneration is the term used to describe the changes in the axon process and supporting Schwann cell layer distal to a site of injury when there is a disconnection of the distal axon from the proximal cell body and axon.[26] The distal axon seals to form an enucleated structure that can still conduct axon potentials for a few days, but disconnection from the nucleus renders it unable to maintain its membrane potential and eventually hydrolysis causes axon rupture.[27] Macrophages clear cellular debris.[28] Endoneurial tubes are cleared of debris and the associated Schwann cells' realign, creating a continuous string of cells called Bungner's bands in preparation for regenerating axons. Later, researchers have defined the role of Schwann cells in providing neurotrophic stimulation to attract regenerating axons and the importance of proteins in the endoneurial tube basement membrane in providing contact adhesion stimuli to the axon for guidance. Dense intraneural scar follows high-energy injuries where there is Schwann cell death, disruption of the endoneurial tubes, and loss of blood supply. Scar forms a physical barrier to nerve regeneration. Low-energy injuries disrupt the axons without excessive damage to the endoneurial tube structure, and there is a greater recovery potential. In preparation for new axonal regeneration, the cell body increases metabolic activity. From the tip of the divided axon, terminal sprouts arise;[29] the most distal part of this sprout is the growth cone, a structure that continually samples the surrounding environment and helps growth along the desired pathway. Filopodia are projections from the cone tip that can extend and retract in minutes.[30] Schwann cell proliferation is necessary for axonal growth. Once contact is made between the axon and Schwann cells, the myelination process begins. Axons which reach a target organ increase in diameter and mature.[31] Axons may grow at rates of 1–3 mm/day. Faster regeneration is seen in low-energy injuries where the injury site is close to the neuron cell body.[32]
Nerve Repair
The traditional technique of suturing two ends of a lacerated nerve is known as an epineural repair.[33] The advantages of this over fascicular repair where every fascicle is separately sutured were described by Daniel and Terzis and include a shorter operating time, technical ease, minimal invasion of intraneural content, and minimal magnification requirements, and the same technique can be used for primary and secondary repairs.
Sunderland emphasized the importance of the nerve ends in a primary repair and suggested preservation of neural blood supply, meticulous handling of the ends, avoidance of tension, and creation of a suitable bed with minimal scar.[1]
Mackinnon demonstrated that immediate primary repair was associated with better outcomes;[34] however, in the acute setting, the injury zone may be difficult to define for nerve ruptures. The forces acting at the repair site are concentrated on the sutures, and excessive tension results in ischemia, distortion of fascicle architecture, scar formation, and a barrier to axon regeneration. Nerves increase in stiffness after injury and excessive tension following nerve debridement or long delays to repair. This can result in failure and gradual repair site supture resulting in the formation of a neuroma in continuity. Multiple small sutures spread the strain across the suture-epineurium interface.[35] Interest is developing in sutureless repair and detensioning repair to attempt to reduce these repair site complications.
Nerve grafting as described by Seddon[20] and later by Millesi[36] is used when primary nerve repair cannot be achieved without excessive tension. This occurs when the injury involves loss of nerve tissue. Without loss of nerve tissue, techniques that allow nerve mobilization, joint positioning, and bone shortening can be used to overcome the elastic retraction caused by the loss of tensegrity.[37] A nerve graft provides a source of endoneurial tubes, which the regenerating axons can follow. Graft revascularization is through vessel growth from proximal and distal nerve stumps adjacent to the graft and from the graft bed. Autologous nerve graft contains some viable Schwann cells; however, migration of viable cells from the adjacent stumps is integral to successful regeneration through long grafts.[38]
The axons within an autologous nerve graft undergo Wallerian degeneration when they are separated from their cell bodies. The nerve is avascular due to disruption of the blood supply. Schwann cells survive for 7 days relying on diffusion;[39] vascular endothelial buds from surrounding tissue beds invade the graft by day 3;[40],[41] large diameter grafts have incomplete revascularization and are at risk of central necrosis.[42]
Nerve Transfer Surgery
In most situations, the site of peripheral nerve injury is the site of direct repair or the site of nerve graft interposition reconstruction.[43] Some nerve injuries, however, cannot be directly repaired. Nerve root avulsions sustained during brachial plexus injury would need reimplantation if the avulsed nerve root back into the spinal cord to enable restoration of continuity from the cell body to the periphery.[44],[45],[46] While technically feasible and a possible strategy for pain management, it carries significant risks to the patient of further nerve injury and no useful functional recovery. In such cases, the concept of nerve transfer surgery has been widely accepted as a superior method of achieving functional motor recovery. The success in brachial plexus injuries has generated interest in the reconstruction of other nerve injuries where although technically possible, the timing, injury, location, and reinnervation distances preclude useful recovery. Proximally sited, high energy disruption of mixed motor-sensory nerve trunks with long injury zones, late presentation, and long reinnervation distances have poor outcomes following autologous nerve grafts. Extending nerve transfer surgery to these situations improves the chances of a functional outcome.
Nerve transfer involves the reinnervation of a distal denervated nerve stump using an expendable motor nerve branch (nerve transfer) or a fascicle from within a main nerve trunk between branch points (highly selective fascicle transfer) directly coapted as close to the denervated muscle. The donor nerve should be in close proximity to the motor point of the denervated muscle to allow a tension-free coaptation with a short reinnervation distance.[47] The process of the muscle reinnervation through the new pathway is called neurotization.
In 1903, the first attempted intraplexal neurotization was performed by Harris and Low. They implanted the distal stump of a ruptured C5 spinal nerve into a healthy C6 spinal nerve.[48] The first documentation of extraplexal neurotization was by Tuttle in 1913.[49] He used branches from the deep cervical plexus to neurotize a ruptured upper trunk. In 1963, Yeoman and Seddon documented intercostal neurotization to restore elbow flexion.[50] Following this in 1972, Kotani et al. described partial spinal accessory nerve transfer to the suprascapular nerve (SSN) in C5/6 avulsion injuries using the lateral branch of the spinal accessory nerve performed through an anterior approach during plexus exploration.[51] In 1984, Narakas[52] documented the concept of neurotization in brachial plexus injury, and the technique of nerve transfer became more widely adopted. The use of the phrenic nerve for neurotization was described by Gu et al. in 1989,[53] and he went on in 1992 to report using the contralateral C7 nerve root as a source of motor axons for transfer following multiple brachial plexus root avulsions.[54] In 1994, Oberlin et al. demonstrated the concept of highly selective fascicle transfer from the ulnar nerve to the motor branch to biceps for elbow flexion restoration in upper trunk rupture or C5 and C6 combined root avulsion injuries. Successful single nerve transfer to innervate the biceps and improve elbow flexion in 1994.[55],[56],[57] Refinements used a double transfer to biceps and brachialis from both ulnar and median nerves, and Mackinnon described the inverse double transfer with the median nerve to biceps and ulnar nerve to brachialis transfer improving the number of reconstructions with Medical research Council (MRC) Grade 4 motor recovery of elbow flexion than that achieved with a single nerve transfer.[58],[59],[60]
The concept of a double muscle restoration with synergistic function achieving greater recovery resulted in Leechavengvongs et al. describing the use of the long head of triceps to reinnervate the anterior division of the axillary nerve for deltoid function as an adjunct to the spinal accessory nerve transfer to the suprascapular nerve in upper brachial plexus injury cases.[61],[62] Reinnervation of both supraspinatus and deltoid improved the shoulder abduction strength and range of motion in a clinical series and systematic review that followed.
In 2003, Witoonchart et al. and Leechavengvongs et al. looked at the feasibility of a posterior approach to the axillary nerve and using the branch to the long head of triceps to provide a nerve transfer.[61],[62] They found the anatomical proximity, diameter, and number of axons to be acceptable. They later presented a 7-case series of upper brachial plexus injuries nerve transfer using the nerve to the long head of the triceps to the anterior branch(es) of the axillary nerve through the posterior approach. The spinal accessory nerve was used simultaneously for nerve transfer to the suprascapular nerve in a double nerve transfer approach. The anterior segment of the axillary nerve was used to prevent axonal loss down the cutaneous sensory branch that arises from the posterior division of the nerve. This double nerve transfer improved functional recovery reinnervating the whole of the axillary nerve including teres minor and infraspinatus, resulting in better external rotation. It became the default reconstruction for C5 avulsion injuries and produced functional recovery.
While this technique provided reliable abduction, shoulder external rotation was poorly reported.[63]
Colbert et al. described a posterior approach using the medial branch of the triceps as it has greater length and more donor axons.[58] The nerve branch to the medial head of the triceps is usually the easiest to expose at the interval between the lateral and long heads. Not only are there greater axon counts than the long head, but it is longer and its harvest does not require intramuscular dissection. It has sufficient length that coaptation can be performed close to the deltoid muscle facilitating rapid reinnervation and allowing for reconstruction where the axillary nerve is ruptured in the quadrangular space.
In their study, they reiterate that teres minor provides two important functions: glenohumeral capsular stability and external rotation. For this reason, they prefer to include the branch of the axillary nerve to teres minor as a recipient in the nerve transfer. They also make no great effort to exclude the sensory branch of the axillary nerve, as studies have shown that donor motor nerves will preferentially seek motor targets over sensory targets.[64]
Despite these refinements, in a proportion of patients, there remains no useful function in the infraspinatus.[65] A possible explanation could be additional damage to the infraspinatus branch of the suprascapular nerve at the spinoglenoid notch sustained at the time of injury that goes unrecognized. Further refinements are suggested with a recent cadaveric study by Nassimizadeh and Power[66] demonstrating a transfer to the infraspinatus using the reinnervated upper lateral cutaneous branch of the axillary nerve as a conduit for direct transfer to the infraspinous branch of the suprascapular nerve or for direct intramuscular neurotization as an adjunct in cases where the suprascapular nerve has been reinnervated but the outcome is uncertain.
In 2014, Leechavengvongs et al. presented an anatomical study showing that the anterior branch of the axillary nerve supplied not only the anterior and middle parts of the deltoid muscle but also the posterior part in most cases (91.5%).[67]
In 2014, Bertelli and Ghizoni described using the medial triceps branch for reinnervation of the anterior division of the axillary nerve through an anterior approach.[68] The advantages reported include the concomitant biceps and brachialis reinnervation through the same incision in C5 and C6 combined injuries, the length of the donor nerve, the inclusion of the branch to the anconeus, and possible better reinnervation of the whole of the deltoid and teres minor.
Between 1972 and 2014, refinements of the technique of neurotization of the shoulder have resulted in widespread adoption of the concept of a double nerve transfer for both shoulder abduction and external rotation with the posterior XI to SSN and the medial triceps to axillary nerve providing the optimum recovery potential.
The technique and innovation described by Bertelli et al. are almost identical to that first described by Adolf Stoffel in 1913, a century earlier.
Adolf Stoffel
Adolf Stoffel was born in Kaiserslautem, Germany in 1880. He studied medicine at the Anatomical Institute, University of Heidelberg and trained under the supervision of Oskar Vulpius, professor of orthopedic surgery. He undertook research into the anatomical distribution of nerve fiber tracts in the central nervous system and the influence of descending tracts on peripheral nerve function. He developed an interest in the surgical management of spastic disorders. He was appointed as an orthopedic specialist in Mannheim, Germany where he continued his research and clinical work into the management of spasticity and paralysis. He studied peripheral nerve anatomy, branching patterns, fascicular topography, and muscle innervation patterns. He developed instruments for peripheral nerve surgery and even a nerve stimulator for using intraoperatively.
In 1911, he published a report on selective partial motor neurotomy for lower limb spasticity with equinovarus foot deformity. In 1913, he published a contemporaneous textbook of orthopedic reconstruction in German entitled “Orthopädische Operationslehre.”[68] A beautifully illustrated chapter “Operationen am nervensystem” describes peripheral nerve branching and fascicular anatomy, with unique nerve transfer procedures that would receive widespread acclaim if published today. Sadly, it was never translated into English from German, perhaps because of its publication immediately before the outbreak of the First World War.
Stoffel described the technique of transfer of a triceps branch to the axillary nerve as described 100 years later by Bertelli et al.[68] [Figure - 1]. He details a method for nerve implantation to another larger nerve [Figure - 2]. He also details the innervation from the axillary nerve, defining the posterior division supply to deltoid, teres minor, and the cutaneous upper lateral cutaneous nerve [Figure - 3].
 |
| Figure 1: Nerve transfer of radial nerve triceps branch into the axillary nerve |
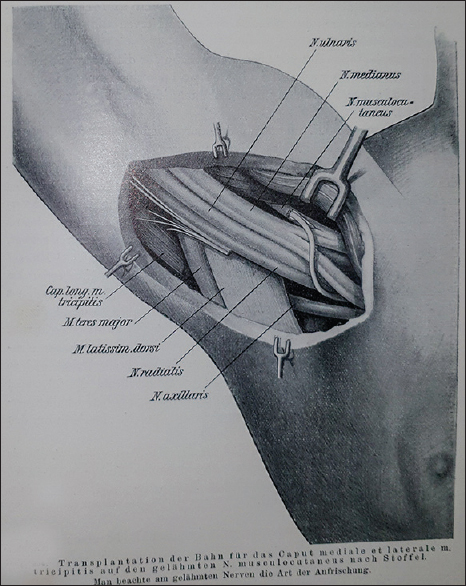 |
| Figure 2: Nerve transfer of radial nerve to musculocutaneous nerve |
 |
| Figure 3: Branching anatomy of the axillary nerve |
This chapter also describes triceps nerve transfer to the musculocutaneous nerve, median to ulnar nerve transfers, gluteal nerve transfers, and possibilities for the management of foot drop [Table - 1].

Stoffel must be credited as the father of modern nerve transfer surgery with the development of innovative surgical techniques for reconstruction of paralysis that have continued to be described for more than a century after his published work.
Conclusion
Nerve transfer surgery has become an essential reconstructive option for the management of nerve injury and paralysis. The reliable results have extended the possibilities for functional reconstruction beyond brachial plexus injuries to complex peripheral nerve injuries. Adolf Stoffel lived from 1880 until 1937 and described fascicle patterns in peripheral nerves and the concept of nerve transfer surgery that he introduced in Germany in the early part of the 19th century. He published his ideas in a textbook in 1913 that failed to reach widespread readership and adoption. The techniques have been redescribed by numerous authors in the ensuing years as the importance of this method of reconstruction became recognized in peripheral nerve reconstruction for paralysis.
George Santayana (1863–1952) stated “Those who cannot remember the past are condemned to repeat it” and sadly, while the intervening world wars progressed our understanding of nerve injury and reconstruction, the outbreak of the First World War just after the publication of “Orthopädische Operationslehre”[69] may have contributed to the relative obscurity of this important surgical textbook that was ahead of its time.
Ethical approval
Ethical approval was cleared by the local Hospital Council.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Authors contributions
MN, AKN and DMP contributed to the literature search, the structuring of the article and overall writing of the paper including proof reading and editing. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Sunderland S. Nerve Injuries and Their Repair: A Critical Appraisal. New York: Churchill Livingstone; 1991. [Google Scholar] |
| 2. | Lanfrancol M. Science of cirurgie. In: Early English Text Society. London: Paul, Trench, Trübner; 1894. [Google Scholar] |
| 3. | Haighton J. An experimental inquiry concerning the reproduction of nerves. Philos Trans R Soc Lond 1795;85:519. [Google Scholar] |
| 4. | Cruickshank W. experimental the nerves, particularly on their reproduction, and on spinal marrow of living animals. Philos Trans R Soc Lond 1795;85:177-89. [Google Scholar] |
| 5. | Haighton J. An experiemental inquiry concerning the reproduction of nerves. Philos Trans R Soc Lond 1795;85:190-201. [Google Scholar] |
| 6. | Muller J. Elements of Physiology. London: Taylor and Walton; 1842. [Google Scholar] |
| 7. | Rawa AL. Ueber die nervennaht. Wien Med Wchnschr 1885;35:358. [Google Scholar] |
| 8. | Letievant JJ. Treated nerve sections. Paris: JB Bailliere et fils; 1873. [Google Scholar] |
| 9. | Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: From Augustus Waller's observations to neuroinflammation. J Peripher Nerv Syst 2002;7:13-27. [Google Scholar] |
| 10. | Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibers. Philos Trans R Soc Lond (Biol) 1850;140:423. [Google Scholar] |
| 11. | Millesi H. Microsurgery of peripheral nerves. Hand 1973;5:157-60. [Google Scholar] |
| 12. | Mafi P, Hindocha S, Dhital M, Saleh M. Advances in peripheral nerve repair techniques to improve hand function: A systematic review of literature, Open Orthop J. 2012;6:60-8. [Google Scholar] |
| 13. | Sherren J. Some points in the surgery of peripheral nerves. Edinb Med J 1906;20:297-332. [Google Scholar] |
| 14. | Mayo-Robson AW. Nerve grafting as a means of restoring function in limbs paralysed by gunshot or other injuries. Br Med J 1917;1:117-8. [Google Scholar] |
| 15. | Mitchell SW. Injuries of Nerves. Philadelphia: Lippincott; 1872. [Google Scholar] |
| 16. | Elsberg CA. Technic of nerve suture and nerve grafting. JAMA 1919;73:1422-7. [Google Scholar] |
| 17. | Langley JN, Hashimoto M. On the suture of separate nerve bundles in a nerve trunk and on internal nerve plexuses. J Physiol 1917;51:318-46. [Google Scholar] |
| 18. | Woodhall B, Beebe G. Peripheral Nerve Regeneration: A Follow up Study of 3656 World War 2 injuries. Washington DC: US Government Printing Office; 1956. [Google Scholar] |
| 19. | Tinel J. The sign of tingling in the lesion of peripheral nerves. Presse Med 1915;23:388-9. [Google Scholar] |
| 20. | Seddon HJ. Surgical Disorders of the Peripheral Nerves. Baltimore: Williams and Wilkins; 1972. [Google Scholar] |
| 21. | Seddon H. Three types of nerve injury. Brain 1943;66:237-388. [Google Scholar] |
| 22. | Sunderland S. Nerves and Nerve Injuries. 2nd ed. New York: Churchill Livingstone; 1978. [Google Scholar] |
| 23. | Sunderland S, Bradley KC. The cross-sectional area of peripheral nerve trunks devoted to nerve fibers. Brain 1949;72:428-49. [Google Scholar] |
| 24. | Rydevik B, Lundborg G. Permeability of intraneural microvessels and perineurium following acute, graded experimental nerve compression. Scand J Plast Reconstr Surg 1977;11:179-87. [Google Scholar] |
| 25. | Buchthal F, Schmalbruch H. Motor unit of mammalian muscle. Physiol Rev 1980;60:90-142. [Google Scholar] |
| 26. | Lundborg G. Nerve regeneration and repair. A review. Acta Orthop Scand 1987;58:145-69. [Google Scholar] |
| 27. | Griffin JW, Hoffman PN. Degeneration and regeneration in the peripheral nervous system. In: Dyck PJ, Thomas PK, Griffin JW, editors. Peripheral Neuropathy. 3rd ed. Philadelphia: Saunders; 1992. p. 361-76. [Google Scholar] |
| 28. | Chaudhry V, Cornblath DR. Wallerian degeneration in human nerves: Serial electrophysiological studies. Muscle Nerve 1992;15:687-93. [Google Scholar] |
| 29. | Morris JH, Hudson AR, Weddell G. A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. I. The traumatic degeneration of myelin in the proximal stump of the divided nerve. Z Zellforsch Mikrosk Anat 1972;124:76-102. [Google Scholar] |
| 30. | Gundersen RW. Sensory neurite growth cone guidance by substrate adsorbed nerve growth factor. J Neurosci Res 1985;13:199-212. [Google Scholar] |
| 31. | Sanders FK, Young JZ. The influence of peripheral connexion on the diameter of regenerating nerve fibres. J Exp Biol 1946;22:203-12. [Google Scholar] |
| 32. | Buchthal F, Kühl V. Nerve conduction, tactile sensibility, and the electromyogram after suture or compression of peripheral nerve: A longitudinal study in man. J Neurol Neurosurg Psychiatry 1979;42:436-51. [Google Scholar] |
| 33. | Karol AG. Peripheral nerves and tendon transfers. Sel Read Plast Surg 2003;9:23. [Google Scholar] |
| 34. | Mackinnon SE. New directions in peripheral nerve surgery. Ann Plast Surg 1989;22:257-73. [Google Scholar] |
| 35. | Giddins GE, Wade PJ, Amis AA. Primary nerve repair: Strength of repair with different gauges of nylon suture material. J Hand Surg Br 1989;14:301-2. [Google Scholar] |
| 36. | Millesi H. Techniques for nerve grafting. Hand Clin 2000;16:73-91, viii. [Google Scholar] |
| 37. | Scarr G. Biotensegrity: The Structural Basis of Life. 1st ed. Handspring Publication Ltd.; 2014. [Google Scholar] |
| 38. | Aguayo AJ, Kasarjian J, Skamene E, Kongshavn P, Bray GM. Myelination of mouse axons by schwann cells transplanted from normal and abnormal human nerves. Nature 1977;268:753-5. [Google Scholar] |
| 39. | Fansa H, Schneider W, Keilhoff G. Revascularization of tissue-engineered nerve grafts and invasion of macrophages. Tissue Eng 2001;7:519-24. [Google Scholar] |
| 40. | Prpa B, Huddleston PM, An KN, Wood MB. Revascularization of nerve grafts: A qualitative and quantitative study of the soft-tissue bed contributions to blood flow in canine nerve grafts. J Hand Surg Am 2002;27:1041-7. [Google Scholar] |
| 41. | Penkert G, Bini W, Samii M. Revascularization of nerve grafts: An experimental study. J Reconstr Microsurg 1988;4:319-25. [Google Scholar] |
| 42. | Best TJ, Mackinnon SE, Evans PJ, Hunter D, Midha R. Peripheral nerve revascularization: Histomorphometric study of small – And large-caliber grafts. J Reconstr Microsurg 1999;15:183-90. [Google Scholar] |
| 43. | Trumble TE, McCallister WV. Repair of peripheral nerve defects in the upper extremity. Hand Clin 2000;16:37-52. [Google Scholar] |
| 44. | Mackinnon S, Dellon AL. Surgery of the Peripheral Nerve. NY: Thieme Medical; 1988. [Google Scholar] |
| 45. | Terzis JK, Sun DD, Thanos PK. Historical and basic science review: Past, present, and future of nerve repair. J Reconstr Microsurg 1997;13:215-25. [Google Scholar] |
| 46. | Naff NJ, Ecklund JM. History of peripheral nerve surgery techniques. Neurosurg Clin N Am 2001;12:197-209, x. [Google Scholar] |
| 47. | Narakas AO, Hentz VR. Neurotization in brachial plexus injuries. Indication and results. Clin Orthop Relat Res 1988;(237):43-56. [Google Scholar] |
| 48. | Harris W, Low VW. On the importance of accurate muscular analysis in lesions of the brachial plexus and the treatment of Erb's palsy and infantile paralysis of the upper extremity by cross-union of nerve roots. BMJ 1903;2:1035-8. [Google Scholar] |
| 49. | Tuttle HK. Exposure of the brachial plexus with nerve transplantation. JAMA 1913;61:15-7. [Google Scholar] |
| 50. | Nagano A, Ochiai N, Okinaga S. Restoration of elbow flexion in root lesions of brachial plexus injuries. J Hand Surg 1992;17:815-21. [Google Scholar] |
| 51. | Kotani PT, Matsuda H, Suzuki T. Trial surgical procedure of nerve transfer to avulsive injuries of the plexus brachialis. Excerpta Med 1972;291:348-9. [Google Scholar] |
| 52. | Narakas AO. Thoughts on neurotization or nerve transfers in irreparable nerve lesions. Clin Plast Surg 1984;11:153-9. [Google Scholar] |
| 53. | Gu YD, Wu MM, Zhen YL, Zhao JA, Zhang GM, Chen DS, et al. Phrenic nerve transfer for brachial plexus motor neurotization. Microsurgery 1989;10:287-9. [Google Scholar] |
| 54. | Gu YD, Zhang GM, Chen DS, Yan JG, Cheng XM, Chen L, et al. Seventh cervical nerve root transfer from the contralateral healthy side for treatment of brachial plexus root avulsion. J Hand Surg Br 1992;17:518-21. [Google Scholar] |
| 55. | Loy S, Bhatia A, Asfazadourian H, Oberlin C. Ulnar nerve fascicle transfer onto to the biceps muscle nerve in C5-C6 or C5-C6-C7 avulsions of the brachial plexus. Eighteen cases. Ann Chir Main Memb Super 1997;16:275-84. [Google Scholar] |
| 56. | Teboul F, Kakkar R, Ameur N, Beaulieu JY, Oberlin C. Transfer of fascicles from the ulnar nerve to the nerve to the biceps in the treatment of upper brachial plexus palsy. J Bone Joint Surg Am 2004;86-A: 1485-90. [Google Scholar] |
| 57. | Oberlin C, Béal D, Leechavengvongs S, Salon A, Dauge MC, Sarcy JJ, et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: Anatomical study and report of four cases. J Hand Surg Am 1994;19:232-7. [Google Scholar] |
| 58. | Colbert SH, Mackinnon S. Posterior approach for double nerve transfer for restoration of shoulder function in upper brachial plexus palsy. Hand (N Y) 2006;1:71-7. [Google Scholar] |
| 59. | Mackinnon SE, Novak CB, Myckatyn TM, Tung TH. Results of reinnervation of the biceps and brachialis muscles with a double fascicular transfer for elbow flexion. J Hand Surg Am 2005;30:978-85. [Google Scholar] |
| 60. | Mackinnon SE. Preliminary results of double nerve transfer to restore elbow flexion in upper type brachial plexus palsies. Plast Reconstr Surg 2006;118:1273. [Google Scholar] |
| 61. | Witoonchart K, Leechavengvongs S, Uerpairojkit C, Thuvasethakul P, Wongnopsuwan V. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part I: An anatomic feasibility study. J Hand Surg Am 2003;28:628-32. [Google Scholar] |
| 62. | Leechavengvongs S, Witoonchart K, Uerpairojkit C, Thuvasethakul P. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part II: A report of 7 cases. J Hand Surg Am 2003;28:633-8. [Google Scholar] |
| 63. | Rohde RS, Wolfe SW. Nerve transfers for adult traumatic brachial plexus palsy (brachial plexus nerve transfer). HSS J 2007;3:77-82. [Google Scholar] |
| 64. | Brushart TM. Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci 1988;8:1026-31. [Google Scholar] |
| 65. | Shin AY, Spinner RJ, Steinmann SP, Bishop AT. Adult traumatic brachial plexus injuries. J Am Acad Orthop Surg 2005;13:382-96. [Google Scholar] |
| 66. | Nassimizadeh M, Power D. Combined Transfer of the Medial Triceps Branch to the Infraspinatus Branch of the Suprascapular Nerve and the Axillary Nerve, and Use of Cutaneous Branch to Reinnervate Infraspinatous, for Restoration of Shoulder Abduction and External Rotation. Paris: FESSH; 2014. [Google Scholar] |
| 67. | Leechavengvongs S, Teerawutthichaikit T, Witoonchart K, Uerpairojkit C, Malungpaishrope K, Suppauksorn S, et al. Surgical anatomy of the axillary nerve branches to the deltoid muscle. Clin Anat 2015;28:118-22. [Google Scholar] |
| 68. | Bertelli JA, Ghizoni MF. Nerve transfer from triceps medial head and anconeus to deltoid for axillary nerve palsy. J Hand Surg Am 2014;39:940-7. [Google Scholar] |
| 69. | Stoffel A. Orthopädische Operationslehre. Stuttgart: F. Enke; 1913. [Google Scholar] |
Fulltext Views
5,341
PDF downloads
2,020





