Translate this page into:
AOCID: More than opinions, evidence
Corresponding Author:
Ayesha Saeed
The Children's Hospital and the Institute of Child Health, Lahore
Pakistan
drayeshasaeed@gmail.com
| How to cite this article: Saeed A. AOCID: More than opinions, evidence. J Musculoskelet Surg Res 2018;2:177-179 |
Dear Editor,
AOCID stands for AO Foundation's “Clinical Investigation and Documentation” institute. It was conceptualized in the early days of the AO Foundation that instrumentation and documentation go hand in hand. In 1999, it was formalized by the concept of AOCID. The AOCID is a unique international team of above 30 specialists, including scientists, medical doctors, engineers, project managers, clinical research associates, data managers, regulatory specialists, quality managers, medical writers, and statisticians, which conducts and publishes high-quality clinical investigations. AOCID is committed to (a) maintain quality and improve efficiency in clinical research and medical affairs services, (b) design and implement registries and multicenter studies, and (c) support surgeons through dedicated clinical research education. In addition, AOCID collaborates with a well-established global network of specialized providers and local clinical research and health economics experts. The AOCID's three functional units, Clinical Operations, Medical Affairs and Health Economics, and Clinical Research Education, are united by the Business Development and Management unit [Figure - 1]. It is AO community's provider of choice for clinical research and medical affairs.
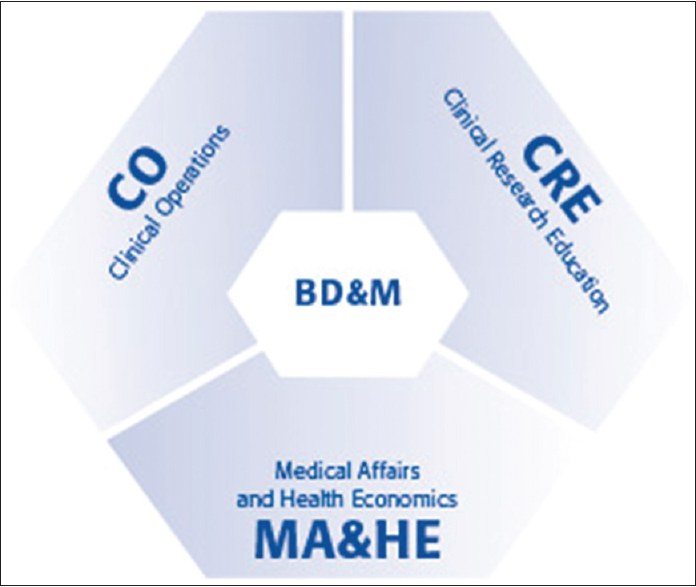 |
| Figure 1: The four integrated units of AOCID |
Since its creation, AOCID has conducted more than 100 clinical studies engaging over 350 clinics in 45 countries and enrolled more than 10,000 patients. Between 2010 and 2016, AOCID has published close to 300 peer-reviewed scientific articles and numerous presentations. In addition to this, around 10 focused registry projects are either running or in development. Over 330 different clinics scattered around the globe have participated in clinical investigations. Since assuming its current form, well over 9000 patients have been recruited to an AOCID study. AOCID performs its tasks in full alignment with ISO 14155, ICH-GCP, and other applicable regulations. An increasing number of outside vendors have audited and appointed AOCID as a “Preferred 3rd Party Provider” in recognition of the expertize housed within.
These impressive numbers form the bedrock of their credo:
“More than Opinions, Evidence.”
Clinical research education for surgeons is another important hallmark of AOCID. It offers services in the form of “Clinical Research Fellowships” and the AO Program for Education and Excellence in Research (AOPEER) online or face-to-face courses. The “Clinical Research Fellowship” program has been established for surgeons to gain training and experience in all phases of clinical research (study planning, monitoring, data analysis, and publication). It is a 3-month duration program for AOTrauma- or AOCMF-sponsored surgeons in which the clinical fellow works on their own projects with the experienced AOCID team under the supervision of a mentor.
AOPEER is a collection of online resources, reference documents, and learning opportunities designed for healthcare professionals who want to learn or improve the skills they need to conduct clinical research. AOPEER aims to empower AO members from all clinical divisions to fill knowledge gaps and gain confidence in their desire to contribute to evidence-based medicine. In 2017 alone, it held nine face-to-face courses including Principles of Clinical Research, Good Clinical Practices, and Grant Writing Course worldwide.
The vision of AOCID is to grow into “A leading clinical research and health economics Institute in trauma and orthopedic surgery.” The AOCID is affiliated with Academia Raetica and Swissmedtech. Their mission statement is “Service to patients worldwide through quality clinical studies, clinical research education, and expertise in health economics.”
Ever since I joined orthopedic residency, I was working on various research projects. However, there was no formal training and guidance regarding research ethics and methodology. At times, it really frustrated me, and I felt that my work lacked quality. Therefore, I decided to pursue some form of formal training. I applied through the website and got selected as the 34th fellow to AOCID. During my fellowship at AOCID, I worked on three projects with my mentor: (i) a systematic review on aneurysmal bone cysts of the pelvis; (ii) a manuscript of a case report on a rare huge aneurysmal bone cyst of the pelvis; and (iii) a clinical investigation protocol on functional outcome of the pediatric neck of femur fractures using pediatric hip plates, which I will complete over a period of 2 years in my home clinic.
The fellowship experience at AOCID exceeded beyond my expectations. As Elke Rometsch (medical writer at AOCID) says, “certain aspects of research are a pain.” During my fellowship, I experienced this aching, throbbing pain quite often, which speaks for the fact that it is designed to improve logical thinking and intellect of the fellows. I learned in AOCID that clinical research is a multidisciplinary teamwork, which when applied to a clinical problem in a systematic manner can generate evidence that impacts human lives. I will never forget the sincere and valuable teachings of Anahi Hurtado (my mentor). Her honest feedbacks improved my work and skills enormously. I am deeply indebted to all my colleagues at AOCID. They were like a family away from home, in the world's most beautiful place. Last but not least, I extend gratitude to AOTrauma for sponsoring the fellowship [Figure - 2] and [Figure - 3].
 |
| Figure 2: During visit to the AOCID office in Davos, Switzerland, Headquarter of AO Foundation |
 |
| Figure 3: Last day at Dubendorf office of AOCID with colleagues |
Thereafter, I have participated as a regional faculty to AOPEER's Basic Principles of Clinical Research Course held in May 2018 in Nepal [Figure - 4]. In July 2018, the AOTrauma Pakistan successfully brought first AOPEER Principle's course to Lahore, Pakistan. It was a well-attended course and was graded high for its usefulness. My journey with AOCID continues [Figure - 5]. I firmly believe that for a sound clinical judgment and an accurate stroke of surgical knife, the intellect and curiosity of a thinking mind are required. This comes with clinical research, and the finest answer to learning this art is coming to “AOCID.” For a robust career in orthopedic research, I strongly recommend to young surgeons to apply for AOCID fellowships.
 |
| Figure 4: May 2018 as Regional Faculty in First AO Program for Education and Excellence in Research Course on Basic Principles of Clinical Research in Kathmandu, Nepal |
 |
| Figure 5: Organized the First AO Program for Education and Excellence in Research Course Basic Principles of Clinical Research in July 2018 in Lahore, Pakistan |
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Further readings
- Available from: https://www.aofoundation.org/Structure/research/clinical-research/Pages/clinical-research.aspx. [Last assessed on 2018 Sep].
- AO Clinical Investigation and Documentation Annual Report; 2017. Available from: https://www.aofoundation.org/Structure/research/clinical-research/Pages/AOCID-News.aspx. [Last assessed on 2018 Sep].
- Available from: https://www.aopeer.org/. [Last assessed on 2018 Sep].
- Available from: https://www.aofoundation.org/Structure/research/clinical-research/activity-service/clinical-research-fellowship/Pages/AOCID-Fellowship-Report-Ayesha-Saeed.aspx. [Last assessed on 2018 Sep].
Fulltext Views
2,506
PDF downloads
1,234





