Translate this page into:
Can we use creatine kinase muscle type as a potential marker for muscle viability in mangled extremities? A preliminary evaluation of its applicability and a literature review
2 Department of Histopathology, PGIMER, Chandigarh, India
Corresponding Author:
Mandeep S Dhillon
Department of Orthopaedics, PGIMER, Sector-12, Chandigarh - 160 012
India
drdhillon@gmail.com
| How to cite this article: Kumar V, Kansal R, Kanojia RK, Vaiphei K, Dhillon MS. Can we use creatine kinase muscle type as a potential marker for muscle viability in mangled extremities? A preliminary evaluation of its applicability and a literature review. J Musculoskelet Surg Res 2019;3:254-259 |
Abstract
Objectives: Increased expression of serum creatine kinase muscle type (CK-MM) in muscle overuse or injury has been documented in many situations. However, the expression of CK-MM and its correlation with the extent of traumatic damage to skeletal muscle tissue has not been explored. We studied the pattern of expression of CK-MM in substantially damaged muscle tissue and attempted to correlate its expression with the extent of muscle damage. Methods: At level 1 trauma center, 15 patients with mangled lower limb were prospectively evaluated. All patients underwent primary amputation (mangled extremity severity score ≥7) using specific criteria. Muscle tissue samples were obtained intraoperatively from 3 different zones (chosen arbitrarily based on clinical parameters of muscle viability, Zones A, B, and C) for all patients. Samples were evaluated by hematoxylin and eosin (H and E) staining and immunohistochemistry (IHC) staining with polyclonal CK-MM antibody. Results: H and E staining correlated with the clinical extent of muscle death in Zones A, B, and C; the percentage of viable muscle fibers was 6.7% in Zone A, 20% in Zone B, and 73% in Zone C. Least CK-MM expression was noted in muscle tissues on IHC in Zone A (most necrotic area) and most in Zone C (most viable area). The score developed by us corroborated with the extent of muscle damage and viability. Conclusion: IHC staining for CK-MM can be used as a definite biomarker of muscle integrity and can be used as an adjunct to clinical evaluation to help define limb viability as well as levels of amputation when that is required.Introduction
In an attempt to improve the understanding of tissue trauma, inflammation, and repair processes, the role of biomarkers has been evaluated over the past few decades.[1],[2],[3],[4],[5],[6],[7],[8],[9],[10],[11],[12] Concerning muscle tissue trauma, most decisions about muscle viability are based on clinicoradiological parameters, predictive scoring systems, and the surgeon's clinical experience. However, in the new millennium, the widening of horizons at the molecular and genetic level has given us the ability to use biomarkers as indirect evidence to predict tissue viability.
Creatine kinase (CK) is one such biomarker that forms an integral part of skeletal muscle; it is a dimeric enzyme, found in both cytosol and mitochondria of muscle fiber. It forms a core functional unit for all chemical reactions and chains occurring in muscle fiber and helps to maintain its structural and functional integrity.[1] Cytosolic form of CK is composed of two subunits, designated as M (muscle type) and B (brain type). These subunits combine to produce three isoenzymes: MM (skeletal muscle), MB (cardiac muscle), and BB (brain tissue); the distribution pattern of these isoenzymes depends on the type of tissue.[2] The skeletal muscle is typically comprised of around 98% CK-MM and 2% CK-MB. In addition, CK-MM accounts for a significant percentage of total and serum CK activity, which is normally found in the serum of healthy adults.[3] The serum level of skeletal muscle enzymes is a potential marker for the functional status of muscle tissue and varies widely in both pathological and physiological conditions. CK activity in the blood is, therefore, believed to be the reflection of muscle membrane integrity.[4]
Various studies have correlated and established the increased expression of CK-MM activity in serum; this is in response to variable insult and injury to the skeletal muscle, more so in athletes.[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17] The existing literature has already defined CK-MM isoform as a marker of myopathies or exercise-induced muscle damage.[5],[6] Even in crush syndrome or prolonged exposure to cold, serum CK activity is markedly increased and can thus be used as a prognostic tool.[7],[8],[9] Some authors had even studied the variable expression of tissue CK activity in marathon runners by skeletal muscle biopsy.[18]
The use of tissue CK activity as a biomarker to predict the extent of muscle damage after the injury has not been mentioned in the published literature in English. We feel that there is some potential in evaluating tissue CK-MM activity after the injury to skeletal muscle tissue, more so in mangled limbs, where damage to muscles is significant, and there is a potential to use this as a prognostic viability tool.
Keeping that in mind, we designed this study in an attempt to evaluate the extent of tissue damage in an indirect way and to see if it is possible to predict tissue healing and survival.
Materials and Methods
This prospective cohort study included 15 patients (aged 18 years or more) presenting with a mangled extremity to the Advanced Trauma Centre of the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, over 1 year. Starting January 2016, patients of age ≥18 years, with both isolated injury and polytrauma, involving either upper or lower extremity and who were planned for amputation based on mangled extremity severity scoring system (≥7), were included for evaluation. Patients suffering from any known autoimmune disorder, immunodeficiency disorder, peripheral vascular disease, vasculitis, diabetes, morbid obesity, or malnourishment were excluded. Approval from the institutional ethical review board was obtained, and informed consent was taken from all participants.
Three different zones in the mangled limb were identified clinically; this was based on the color, consistency, contractility, and morphology of involved muscles. Zone A comprised of muscles from the actual mangled part of the limb, Zone B was the intermediate zone between Zones A and C with the tissue of doubtful viability, whereas Zone C consisted of proximal healthy and viable muscle through which the final resection of the limb was done. Skeletal muscle samples were taken from each zone separately [Figure - 1]. All the biopsy samples from these patients were stored in 10% buffered formalin and processed further. Hematoxylin and eosin (H and E)-stained sections were used for the baseline study of the cases; interpretations with respect to tissue viability, integrity, and inflammation were done; and the special comment was made on the extent of degenerated muscle fibers with altered morphology and intercellular edema with interspersed inflammatory cells. Immunohistochemistry (IHC) was carried out by peroxidase antiperoxidase method using two micron-thick paraffin sections mounted on poly-l-lysine-coated slides. Rabbit polyclonal antibody in a dilution of 1:200 was used, after standardization of antibody.
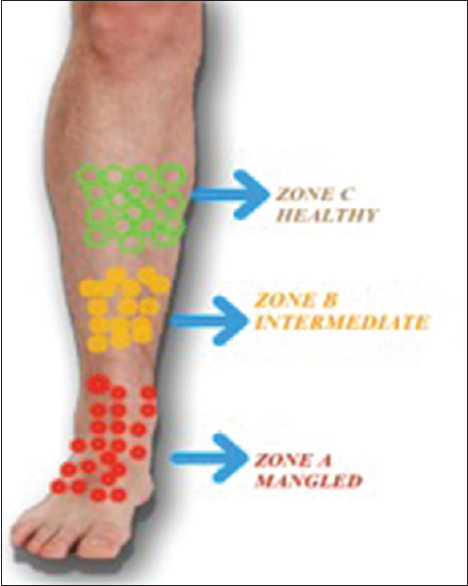 |
| Figure 1: Pictorial representation of different zones chosen intraoperatively. The boundary between various zones was taken arbitrarily, based on clinical discrimination of healthy and injured tissue |
The CK-MM antibody stains only intact and viable muscle fiber. Therefore, staining of CK-MM was expected to stain viable tissue only. The scoring of CK-MM was designed to evaluate the loss of stain intensity and pattern of involvement of muscle fibers; samples with less viable tissue were expected to have higher combined CK-MM scores and more loss of staining.
Loss of the staining was interpreted in context to normal control muscle, and the degree of staining was added to the evaluation, leading to a special 2-part scoring system as follows:
- The pattern of muscle fiber involvement was scored into three types subjectively:
- Only scattered individual fibers showed a loss of staining
- A larger group of muscle fibers showed a loss of staining
- Very large group or whole muscle fascicle showed loss of staining.
- The intensity of staining retained by the affected muscle fibers was scored into three types subjectively:
- A mild degree of loss of staining
- A moderate degree of loss of staining
- Total loss of staining.
The two scores were added up for the combined score of CK-MM for each biopsy. Thus, the minimum combined score of 2 meant that only scattered individual muscle fiber showed a mild degree of loss of staining (good viability, good prognosis). The highest possible combined score of 6 was interpreted as a large group or the whole fascicle, showing a total loss of staining (poor viability, poor prognosis).
The data obtained were analyzed using appropriate statistical tests. All discrete categorical data were represented in the form of either a number or percentage, whereas the continuous data, which were normally distributed, were written in the form of its mean and standard deviation. If the data were skewed (ordered categorical data), it was represented in the form of its median and interquartile range. The normality of quantitative data was checked using measures of Kolmogorov–Smirnov tests of normality. The Mann–Whitney test was employed when two groups of skewed data were under consideration. In the case of normally distributed data, the means of two groups were compared using independent t-test. Proportions were compared using either the Chi-square test or Fisher's exact test, depending on their applicability. McNemar test was applied for comparison between categorical values of different zones. Whereas, the Wilcoxon signed-rank test was used for categorical data. All the statistical tests were two-sided and were performed at a significance level of α = 0.05. The statistical analysis was performed using IBM SPSS Statistics (version 22.0, IBM corportaion, India).
An extensive literature search was done on PubMed [Table - 1] using the following search string (“creatine kinase”[MeSH Terms] OR (“creatine”[All Fields] AND “kinase”[All Fields]) OR “creatine kinase”[All Fields]) AND (mangled [All Fields] AND (“extremities”[MeSH Terms] OR “extremities”[All Fields] OR “extremity”[All Fields]) but no relevant literature could be traced. Further literature search with different MeSH term (“creatine kinase”[MeSH Terms] OR (“creatine”[All Fields] AND “kinase”[All Fields]) OR “creatine kinase”[All Fields]) AND ((“crush injuries”[MeSH Terms] OR (“crush”[All Fields] AND “injuries”[All Fields]) OR “crush injuries”[All Fields] OR (“crush”[All Fields] AND “injury”[All Fields]) OR “crush injury”[All Fields]) AND (“extremities”[MeSH Terms] OR “extremities”[All Fields] OR “limb”[All Fields]), 22 articles were identified. After detailed screening of abstracts and bibliography, no relevant study regarding CK-MM expression in skeletal muscle in crushed or mangled limb could be found.

Results
The average age of patients was 40 years (range 20–70 years), and 2 of the 15 patients were females. All patients were involved in major accidents, with road traffic accidents being the most common mode of injury [Table - 2].
On examination of H and E-stained tissue sections with CK-MM antibody, the following microscopic findings were noted.
Hematoxylin and eosin-stained sections
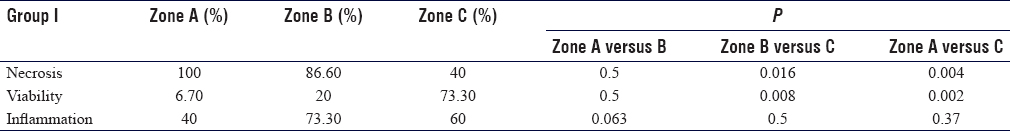
Sections from Zone A showed diffuse necrosis in 100% samples. In Zone B, 86.6% of sections had necrotic muscle fibers, which was further reduced to 40% in Zone C. This decrease from Zone A to C and from Zone B to C was statistically significant, with P = 0.004 and 0.016, respectively. Likewise, 6.7% of sections in Zone A had traces of viable muscle fibers which increased to 20% in Zone B and 73% in Zone C. This increase from Zone A to C and Zone B to C was also statistically significant with P = 0.002 and 0.008, respectively [Table - 3].
Immunohistochemistry evaluation of creatine kinase-skeletal muscle
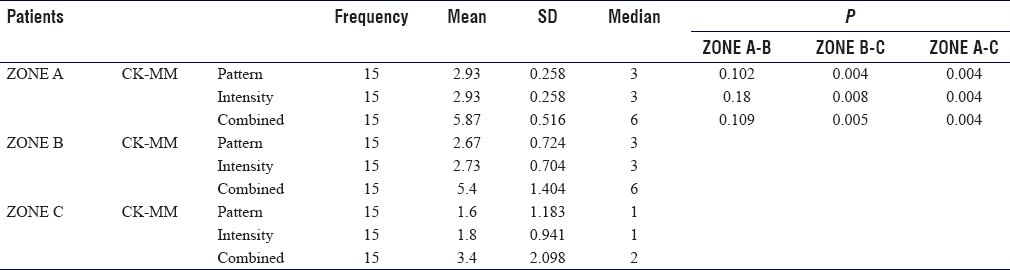
In Zone A, large muscle fascicles with complete loss of staining were noted with a median combined Score 6 with a range of 2–6. In Zone B, a large group of muscle fascicles with moderate to complete loss of staining were noted with a median combined Score 6 with a range of 2–6. In Zone C, muscle fibers with mild to moderate loss of staining were noted with combined Scores of 2 and 3, having a median Score of 2 [Figure - 2]a and [Figure - 2]b.
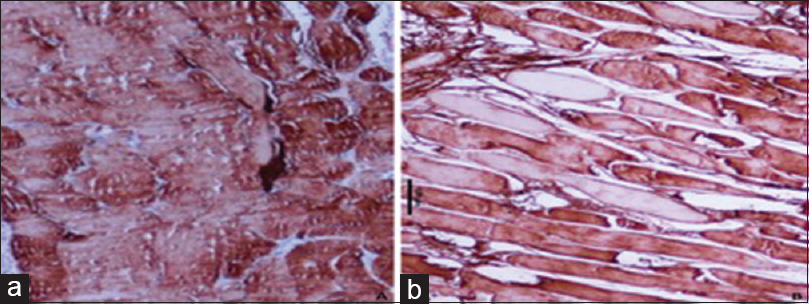 |
| Figure 2: Photomicrographs showing the different intensity of immunohistochemistry staining for creatine kinase-MM in degenerated muscle, (a) injured muscle showing mild degree loss of creatine kinase-MM proteins by a large number of muscle fibers (peroxidase antiperoxidase, ×400), (b) injured muscle showing a total loss of staining for creatine kinase-MM protein by just a few scattered muscles (peroxidase antiperoxidase, ×400) |
In Zone A and B, the median CK-MM score for both pattern and intensity was 3 with a range of 1–3, and for a combined score, it was 6 with a range of 2–6. Whereas for Zone C, it was 1 with a range of 1–3 for both pattern as well as the intensity, while the combined CK-MM score was 2 with a range of 2–6 respectively. On the application of the Wilcoxon signed-rank test, this decrease from Zone A to C and from Zone B to C was found statistically significant. This implies that the staining of CK-MM increased from Zone A to Zone C, while the CK-MM scores increase from Zone C to A [Table - 4]. Further, IHC staining was noted to be more sensitive than H and E stains, as those myofibers that appeared normal on H and E sections showed some focal loss of staining and involvement of a few muscle fibrils.
Inference
CK-MM scores were higher in Zone A and lowest in Zone C. Higher scores were observed in those sections where a large group of fascicles had significant loss of CK-MM stains. These observations were corroborating with H and E stain findings. In Zone A (mangled part), a higher proportion of necrotic tissues corresponds to higher CK-MM scores, while in Zone C, as the proportion of viable muscle fibers increases, the CK-MM scores decrease.
Discussion
In the present study, we were able to demonstrate a substantial increase in viable muscle fibers (6.7%–73%) from Zone A to C on the H and E examination [Table - 3]. These observations further corroborated with observations of IHC staining with the CK-MM antibody. Using our arbitrary division into 3 zones, higher scores of CK-MM were noted in Zone A (with more percentage of nonviable fibers) compared to lower CK-MM scores in healthy muscle zone (Zone C) [Table - 4]. Our preliminary data point toward CK-MM being a more sensitive marker for muscle viability compared to H and E staining alone; this allows the surgeon more insight into options of limb salvage when the decision is problematic.
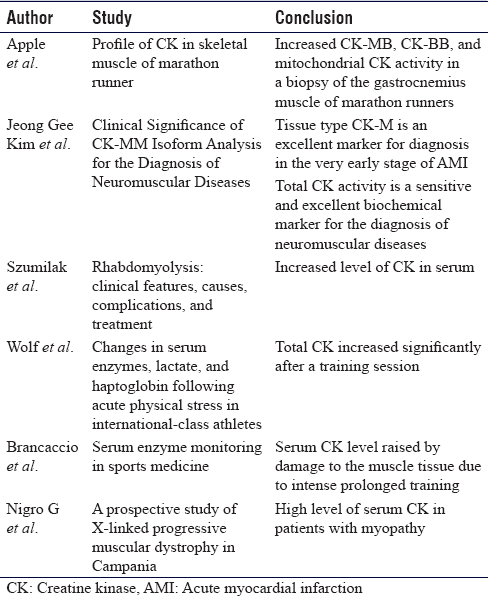
Sufficient evidence is recorded in the literature showing altered expression of serum CK-MM in the event of acute muscle insult, either in the form of direct or indirect injury.[5],[6],[7],[8],[9],[10],[11],[12],[13],[14],[15],[16],[17] Wolf et al. had demonstrated a rise in serum CK-MM levels, along with lactate, in association with acute physical stress in international athletes.[15] Similar findings were reported by Brancaccio et al. in 2008.[6] Most of the published literature has focused on serum CK activity, and only a few have studied the altered expression of CK in muscle tissue in the event of injury [Table - 5]. Apple et al. subsequently studied the profile of CK-MM isoenzyme in skeletal muscles in marathon runners and found an increase in CK-MB, CK-BB, and mitochondrial CK, postmarathon running on skeletal muscle biopsy.[18] This naturally evolves into a thought process that brings into focus the correlation of this enzyme with acute skeletal muscle trauma, with the possibility of the predictive value of tissue viability. To date, no English literature has explored the role of tissue CK-MM in predicting muscle viability, particularly in mangled injuries [Table - 1]. This study has been initiated as a pilot project to explore and validate the role and utility of tissue CK-MM as a biomarker in such high-velocity trauma.
Although our data point toward CK-MM staining having a potential role as a predictor of muscle viability and integrity, it is also important to note that those tissue sections that show normal morphology on H and E stains have shown the variable intensity of CK-MM loss on IHC staining. This probably indicates the high sensitivity of this biomarker in tissue for muscle viability and needs further research and corroboration in bigger patient subsets. Our preliminary work points toward the potential role of CK-MM in predicting the tissue salvageability, by delineating the nonviable tissue, but the clinical correlation is important.
The main limitations are the high cost and quantity of antibody, and the current study has a limitation of small sample size. Furthermore, the 2-part scoring system devised and used for CK-MM is unique and quite subjective. Larger studies, preferably multicentric, may give us more sound insights into this as a predictor of muscle viability in the future.
Conclusion
Preliminary data point toward CK-MM being a potential marker of muscle integrity, as it is a good indicator for identifying nonviable muscle; this may not be obvious in HE-stained sections. The advantage of CK-MM having differential expression in different zones of muscle injury alludes to its potential role as a predictor for amputation.
Recommendation
The study highlights the role of tissue CK-MM as a biomarker, by defining the extent of skeletal muscle trauma by delineating viable and nonviable muscle fibers. Large multicenter trials are recommended to consolidate these results and to further explore the clinical applicability of this biomarker in day-to-day practice. The current study is an attempt to widen the horizons for better understanding and management of mangled injuries in the nearby future.
Ethical consideration
This study was approved by the review board committee of the PGIMER, Chandigarh, India. The study strictly adhered to guidelines of the Indian Council of Medical Research (ICMR), 1994 and Helsinki declaration of 1975, revised in 2000.
Acknowledgment
This study was partly funded by a research grant cell of the ICMR, Department of Health Research, New Delhi.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Author's contribution
RKK, VK, and RK conceived and designed the study. KV and RK provided research material, collected, and organized data. KV and RK analyzed and interpreted the data. MSD, VK, and RK contributed to the definition of intellectual content and wrote initial and final drafts of the article. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Wallimann T, Hemmer W. Creatine kinase in non-muscle tissues and cells. Mol Cell Biochem 1994;133-134:193-220. [Google Scholar] |
| 2. | Wevers RA, Olthuis HP, Van Niel JC, Van Wilgenburg MG, Soons JB. A study on the dimeric structure of creatine kinase (EC 2.7.3.2). Clin Chim Acta 1977;75:377-85. [Google Scholar] |
| 3. | Baird MF, Graham SM, Baker JS, Bickerstaff GF. Creatine-kinase-and exercise-related muscle damage implications for muscle performance and recovery. J Nutr Metab 2012;2012:960363. [Google Scholar] |
| 4. | Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc 1992;24:512-20. [Google Scholar] |
| 5. | Nigro G, Comi LI, Limongelli FM, Giugliano MA, Politano L, Petretta V, et al. Prospective study of X-linked progressive muscular dystrophy in Campania. Muscle Nerve 1983;6:253-62. [Google Scholar] |
| 6. | Brancaccio P, Maffulli N, Buonauro R, Limongelli FM. Serum enzyme monitoring in sports medicine. Clin Sports Med 2008;27:1-18, vii. [Google Scholar] |
| 7. | Malinoski DJ, Slater MS, Mullins RJ. Crush injury and rhabdomyolysis. Crit Care Clin 2004;20:171-92. [Google Scholar] |
| 8. | de Meijer AR, Fikkers BG, de Keijzer MH, van Engelen BG, Drenth JP. Serum creatine kinase as predictor of clinical course in rhabdomyolysis: A 5-year intensive care survey. Intensive Care Med 2003;29:1121-5. [Google Scholar] |
| 9. | Liu J, Zhang Z, Yang Z, Li F, Yan P, Liu Y. Effects of freezing and hypoxia on serum creatine kinase activity in rats. Space Med Med Eng (Beijing) 1996;9:291-4. [Google Scholar] |
| 10. | Munjal DD, McFadden JA, Matix PA, Coffman KD, Cattaneo SM. Changes in serum myoglobin, total creatine kinase, lactate dehydrogenase and creatine kinase MB levels in runners. Clin Biochem 1983;16:195-9. [Google Scholar] |
| 11. | Nakamura N, Uzawa R, Ishii T. Serum creative kinase-MM sub-bands. The healthy value of CK-MM sub-bands in serum and changes in CK-MM sub-bands in acute myocardial infraction. J Sltowa Med Assoc 1984;44:331-6. [Google Scholar] |
| 12. | Wevers RA, Delsing M, Klein Gebbink JA, Soons JB. Post-synthetic changes in creatine kinase isozymes (EC 2.7.3.2). Clin Chim Acta 1978;86:323-7. [Google Scholar] |
| 13. | Szumilak D, Sułowicz W, Walatek B. Rhabdomyolysis: Clinical features, causes, complications and treatment. Przegl Lek 1998;55:274-9. [Google Scholar] |
| 14. | Mokuno K, Riku S, Sugimura K, Takahashi A, Kato K, Osugi S. Serum creatine kinase isoenzymes in duchenne muscular dystrophy determined by sensitive enzyme immunoassay methods. Muscle Nerve 1987;10:459-63. [Google Scholar] |
| 15. | Wolf PL, Lott JA, Nitti GJ, Bookstein R. Changes in serum enzymes, lactate, and haptoglobin following acute physical stress in international-class athletes. Clin Biochem 1987;20:73-7. [Google Scholar] |
| 16. | Priest JB, Oei TO, Moorehead WR. Exercise-induced changes in common laboratory tests. Am J Clin Pathol 1982;77:285-9. [Google Scholar] |
| 17. | Ide M, Tajima F, Furusawa K, Mizushima T, Ogata H. Wheelchair marathon racing causes striated muscle distress in individuals with spinal cord injury. Arch Phys Med Rehabil 1999;80:324-7. [Google Scholar] |
| 18. | Apple FS, Rogers MA, Sherman WM, Costill DL, Hagerman FC, Ivy JL. Profile of creatine kinase isoenzymes in skeletal muscles of marathon runners. Clin Chem 1984;30:413-6. [Google Scholar] |
Fulltext Views
3,008
PDF downloads
1,347





