Translate this page into:
Effectiveness of single injection of platelet-rich plasma over corticosteroid in the treatment of plantar fasciitis – A randomized, comparative study
Corresponding Author:
Pabitra K Sahoo
Swami Vivekananda National Institute of Rehabilitation Training and Research, Olatpur, Cuttack - 754 010, Odisha
India
pabitra2406@gmail.com
| How to cite this article: Sahoo PK, Ujade NA, Das SP. Effectiveness of single injection of platelet-rich plasma over corticosteroid in the treatment of plantar fasciitis – A randomized, comparative study. J Musculoskelet Surg Res 2020;4:187-193 |
Abstract
Objectives: Plantar fasciitis (PF) is not an uncommon cause of heel pain whose treatment is not yet standardized. Although platelet-rich plasma (PRP) and corticosteroid (CS) injections are the two commonly used modalities, yet not much importance has been given to the comparison of their roles in sustained functional improvement. We aimed to study the effect of PRP and CS injections in PF and compare their effectiveness with respect to pain relief and improvement of functional and patient satisfaction. Methods: Seventy-three cases were randomized into two groups: 39 patients (Group A) received a single injection of autologous PRP and 34 in Group B received a single injection of CS (40 mg of methylprednisolone) by the random selection. A structured home exercise program was demonstrated to both the groups, as baseline management. The effectiveness was assessed and compared in preinjection and postinjection at 3- and 6-months follow-up. Visual Analog Scale (VAS), Roles and Maudsley (RM), and Foot Function Index (FFI) scoring systems were used as outcome measures. Results: CSs had an early effect, reducing pain to a moderate level in 82.4% of patients compared to PRP (P = 0.000). However, the effect was not sustainable over a long period. On the other hand, PRP was found to have better pain relief over 3 months and 6 months follow-up with a mean VAS score of 2.0 ± 0.9 and 0.8 ± 0.8, respectively (P = 0.000). There was a significant improvement of FFI and RM score as well as at 6 months follow-up (P = 0.000). Conclusion: Injection of CS had an early effect, which is not sustainable, whereas PRP was found to have a prolonged impact on pain relief and better patient satisfaction with treatment outcomes. Therefore, PRP can be advised for sustained and prolonged improvement in PF.
Introduction
Several terminologies were used to describe pain at the plantar surface of the heel, which includes policeman's heel, heel spur syndrome, joggers heel, sub-calcaneal pain, plantar heel pain, plantar fasciopathy, plantar fasciitis (PF), and plantar fasciosis.[1] The incidence of PF varies from 3.83 to 10.5/1000 population per year, with a higher incidence in females.[2],[3] Increasing age and high body mass index are the factors associated with a higher incidence of heel pain.[4] Therapeutic modalities such as extracorporeal shock-wave therapy (ESWT), plantar fascia and Achilles tendon stretching exercises, night splints, shoe inserts and medical managements such as nonsteroidal anti-inflammatory drugs (NSAID), local corticosteroid (CS) injection, platelet-rich plasma (PRP) injection, and prolotherapy are used for the treatment of PF.[5] No consensus has been reached to make out the most effective modality. Moreover, the outcomes of different studies are inconsistent.[6] Although clinicians often use CS and PRP to treat chronic PF, the outcome of CS lacks high-level evidence for reliability.[7] Cochrane database of a systematic review comparing local steroid injection with placebo or no treatment in treating PF has shown a slightly reduced heel pain up to 1 month only.[8] A systematic review and meta-analysis also have shown no difference in pain or function score at long-term follow-up.[9] PF is considered a cumulative trauma disorder involving a degenerative process rather than inflammation. Therefore, PRP, having the potential for tissue regeneration, is theoretically superior to CS.[10] The anti-inflammatory and regenerative properties of PRP have been proved by several studies.[11],[12] The literature on the efficacy of various treatment modalities for PF shows conflicting results. Although many studies have used PRP and CS in PF management, very few of them, have compared their role in terms of functional improvement and patient satisfaction.
The objective of the current study was to analyze the effectiveness of single PRP injection over CS injection in chronic PF combined with a structured home exercise program as baseline management and comparison of their efficacy in terms of pain relief and functional improvement. The study being conducted at a National Level Research Institute having divergent patient characteristics, the results will improve the level of evidence in evaluating the efficacy of the above two modalities.
Materials and Methods
This interventional study was conducted at a National Rehabilitation Training and Research Institute, India, from November 2017 to December 2019. Patients with heel pain at first steps in the morning or after a period of rest and sharp pain with the palpation of the medial plantar calcaneal region, aggravated with ankle and great toe dorsiflexion, were diagnosed to have PF. Those patients between 18 and 60 years of age who did not respond to a minimum of 3 months of conservative treatment, including analgesics, stretching exercises, and night splint, were included in the study. Those with a history of rheumatoid arthritis, gout, degenerative arthritis, neural entrapment syndromes, bleeding disorders, skin lesion on heel, pregnancy, malignancy, calcaneodynia secondary to injury or fracture, and cases with a prior history of local injection or any intervention within 6 months were excluded from the study. Patients with uncontrolled diabetic mellitus, anemia, low cognitive status, and those received NSAID 1 week before the study were also excluded.
Assuming that the patients presenting in the outpatient department randomly, every alternate patient was allotted to Group A, who were administered a single dose of autologous PRP Injection, and Group B, who received a single dose of CS (methylprednisolone) injection following simple randomization procedure, until the minimum sample size was met.
The outcome measures used were the Visual Analog Scale (VAS) score for pain, Roles and Maudsley (RM) score for pain on walking and patient satisfaction, and Foot Function Index (FFI) score for functional improvement. Scores were recorded before injection, at 3- and 6-month follow-up. VAS scores of patients were also recorded at 5 hours post-injection, just before leaving the hospital. The sample size determination has been done for the Chi-square test of independence using G*Power 3.1.9.2 statistical power analysis with a bio statistician's help. The minimum sample size came out as 69 to achieve the power of the test of 0.80 for 0.05 level of α. A total of 78 patients were enrolled for the study, out of which 5 patients were lost to follow-up. Therefore, the final sample size was 73.
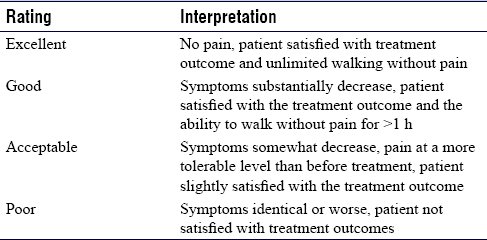
The intensity of plantar heel pain was measured by VAS using a ruler with anchor points 0 as no pain 10 as the worst possible pain.[13],[14] It was further classified as no pain (0), mild pain (1–3), moderate pain (4–6), and severe pain (7–10). Modified RM score was used to assess patient satisfaction and limitation of walking ability due to pain [Table - 1].[15],[16],[17] The function in terms of pain, disability, and activity restriction was measured using FFI, which is a patient-related outcome questionnaire consisting of 23 items, divided into three subscales.[18]
A double-centrifugation technique was used for the preparation of PRP. Around 15 ml of autologous peripheral venous blood was collected atraumatically, avoiding platelet activation and anticoagulated with 1.5 ml sodium citrate. Initial platelet count was done for peripheral blood. Red blood cells were separated by the first centrifugation done at 2500 rpm for 15 min, followed by 3000 rpm for 5 min to obtain a plasma sample having a higher concentration of platelet, known as PRP. The total platelet count was compared with the initial platelet count. Around 3 ml pure PRP was obtained from the deeper layer and was injected immediately in the plantar fascia of group A patients. CS solution was prepared with 40 mg of methylprednisolone and 1 ml of 2% lignocaine and injected locally in Group B patients.
A standard injection technique was followed for injection into the plantar fascia.[19] The medial heel was exposed with external rotation of the affected limb. The PRP or CS was injected using a 25 G needle directing laterally on the plantar surface, just superior and anterior to calcaneus till it touches the periosteum. Care was taken to avoid injecting into the plantar fat pad. A home exercise program for plantar fascia and Achilles tendon stretching was demonstrated and explained to both groups (three sets of each exercise for 10 min duration with 10 repetitions in each set).[20]
Statistical analysis
The data analysis was done using IBM SPSS Statistics, 24.0 software (IBM corp., Bio-statistician). The association of categorical variables such as VAS, RM, and FFI scores classified according to Group A and B and studied using the Chi-square test of independence. Computation of mean of VAS and FFI scores at different time intervals was done following descriptive statistics procedure and comparing their means between the two groups using the nonparametric Mann–Whitney U-test. For the statistical test of significance, a cut off “p” value was taken as <0.05.
Results
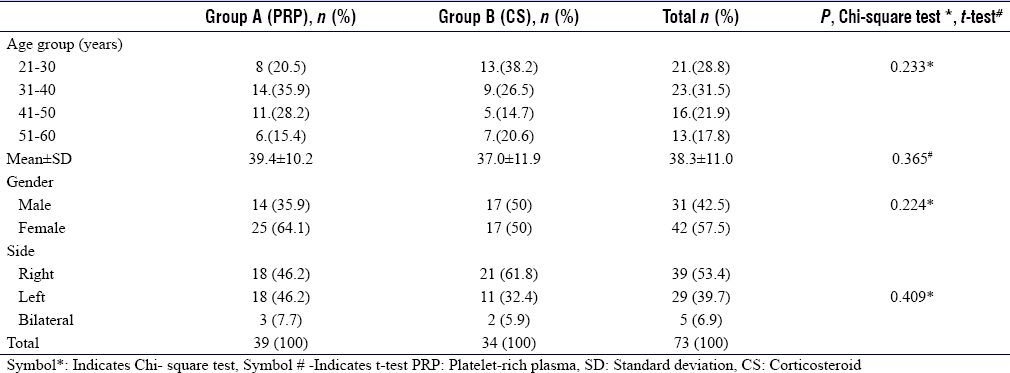
Out of 73 patients, 39 belonged to Group A and 34 to Group B. The age, gender, and laterality distribution are summarized in [Table - 2]. The demographic criteria of patients in both the groups of the current study are comparable with other studies.[13],[14],[21],[22],[23] In patients with bilateral PF, the intervention was done in the most painful side. The Chi-square test of independence revealed no significant association between age distribution, gender distribution, and laterality between the two groups (P = 0.233, P = 0.224, and P = 0.409, respectively).
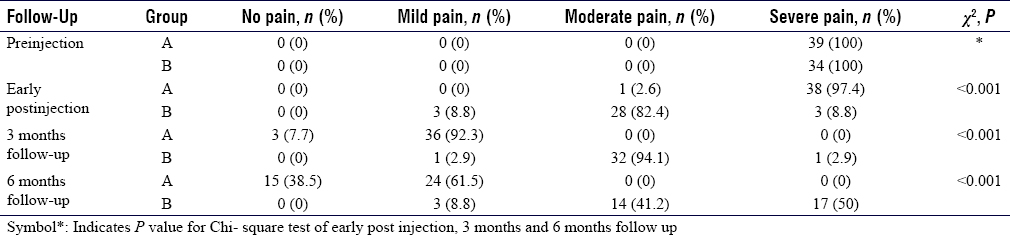
At 5 hours of postinjection, 97.4% of Group A and only 8.8% of Group B had preinjection pain (P = 0.000), with a mean VAS score of 8.0 ± 0.7 and 5.2 ± 1.2, respectively. At 3 months, the majority in Group A had mild pain (92.3%), whereas Group B had moderate pain (94.1%). Recurrence of severe pain (mean VAS 6.3) was observed in half of the Group B patients at 6 months, whereas all patients in Group A improved to either mild or moderate pain (P = 0.000) [Table - 3].
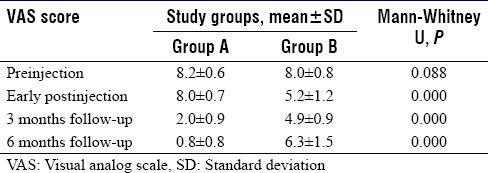
Group A has shown a significantly lower mean VAS score than Group B, both at 3 and 6 months (P = 0.000) [Table - 4]. Comparison of pain level and mean VAS score at different time intervals implied that Group A had no early pain relief, but there was substantial relief at 3 and 6 months. There was some pain relief at the early post-injection in Group B, which became remarkable at 3 months. However, the effect was not sustained, rather pain severity increased at 6 months, remaining below the preinjection level [Figure - 1].
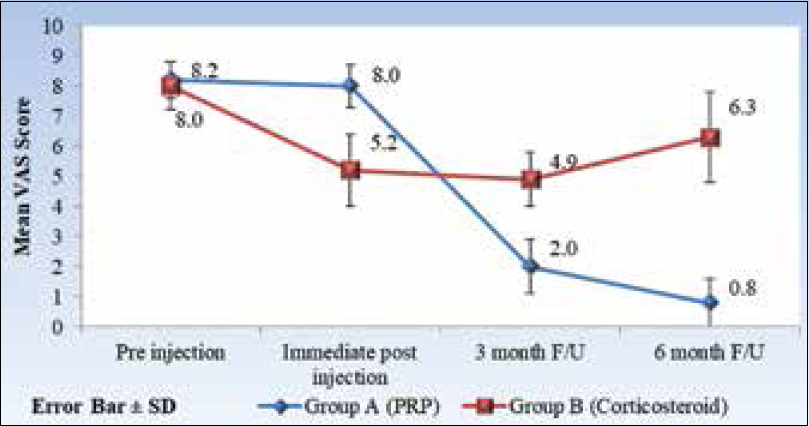 |
| Figure 1: Comparison of mean visual analog scale score at different time intervals between the two treatment groups |
Comparison of Roles and Maudsley score
At preinjection, both groups had low or fair RM score without significant difference (P = 0.442). After the intervention, a significant difference was observed in RM scores among two groups at 3 and 6 months with P = 0.026 and P = 0.000, respectively. Fair to good functional improvement was observed at 3 months in both groups. At 6 months, Group A showed significantly better function in terms of movement and patient satisfaction [Table - 5].
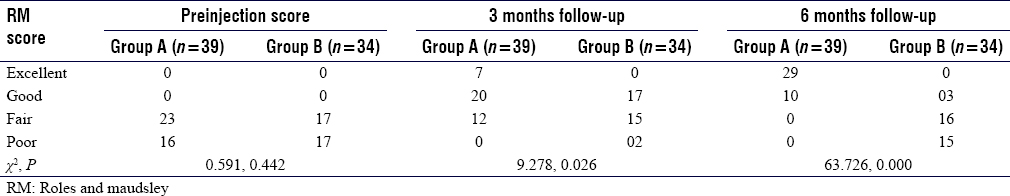
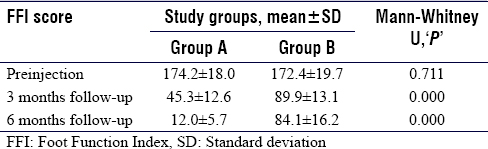
Comparison of functional improvement with Foot Function Index score
The comparison of mean FFI score at the different time intervals between the two treatment groups has shown that the mean FFI score of both groups has reduced considerably at 3- and 6-month follow-up (P = 000). However, the mean FFI scores in Group A were significantly lower than Group B [Table - 6]. No adverse events were noticed in any of the groups.
Discussion
The present study revealed that the pain relief of most of the CS (Group B) patients has come down from severe to moderate pain at the early postinjection stage, while at 3 months and 6 months follow-up, the effectiveness was significantly better with the injection of PRP. Most of these patients were satisfied with the treatment outcome having unlimited walking without pain. The early effect of both injections was measured after 5 hours of postinjection, just before the patient left the hospital. Lignocaine used with CS has a rapid onset of local anesthetic effect lasting for up to 30–60 min in its plain form.[24] Since the excretion half-life of Lignocaine is for 90–110 min,[24] the effect assessed after 5 hours postinjection was more of CS. The use of Lignocaine with PRP was avoided as it could directly interfere with platelet functionality, especially platelet aggregation.[25] Moreover, PRP injection combined with local anesthetic is less effective than PRP injection alone.[26] The PRP action on plantar fascia regeneration, pain relief, and functional improvement have been described by different authors in different ways. Some authors postulated PF to be a chronic degenerative condition due to repeated microtrauma associated with the formation of angiofibroblastic tissue.[13] In chronic cases, due to cumulative trauma, the micro-tears at its attachment cannot heal, leading to collagen denaturation. A degenerative mechanism in PF is established with histological findings such as collagen necrosis, chondroid metaplasia, and calcification.[27] Hypovascularity of plantar fascia prevents accessibility to a high concentration of platelet and other growth factors for natural repair. Injection of PRP directly delivers platelets into the lesion, which releases, platelet-derived growth factor, transforming growth factor beta, endothelial growth factors, that accelerate tissue healing. The anti-inflammatory and antinociceptive effects of PRP are well established in the literature.[28],[29] Injection of PRP in Group A patients of the current study has shown an antinociceptive effect by relief of pain and functional improvement at 3- and 6-month follow-up. The meta-analysis by Chen et al.[30] has shown significant pain relief at 1.5 and 3 months in the CS group but sustained pain relief in the PRP group at 12 weeks, which is also reflected in the current study. Patient satisfaction and functional scores give a significantly better edge to patients in group A. A similar result was observed in a meta-analysis by Yang et al.[27] with better long-term pain relief after 24 weeks. However, no significant difference in the RM score and foot function score was observed among both groups. Our study results on the sustained effects of PRP over CS is supported by other studies.[13],[17],[21],[22],[31] The review article by Monto[32] suggested that PRP's effect can be considered an alternative to surgical care in case of severe chronic PF. A comparative study between PRP and CS by the same author[33] showed initial pain and function improvement scores at 3 months in the CS group, followed by a drop down to the baseline at 12 months. In contrast, the PRP group showed a sustained improvement beyond 24 months. A similar inference was drawn by Ling and Wang[34] in the meta-analysis of 10 randomized control trials involving 445 patients except for the fact that the RM score on subgroup analysis with control regimen did not show any advantage of CS. The current study has shown a comprehensive outcome, including pain, disability, activity limitation, and overall patient satisfaction comparing two treatment groups at different follow-up intervals.
Martinelli et al. studied the safety and efficacy of PRP in PF[15] with three PRP injections at weekly intervals. The RM score at 12-month follow-up was excellent in 64% cases, and no adverse events were noted with repeated PRP injections. In the current study, excellent RM score has been observed at the end of 6 months follow-up in 74% of cases administered with a single PRP dose.
The systematic review and meta-analysis by Whittaker et al.,[7] which included 47 trials, supports CS injection as an effective treatment over other comparators for pain relief and functional improvement. Other systematic reviews and meta-analysis conducted by Singh et al.[9] and the study by Jain SK[35] reported no difference in pain or functional score at long-term follow-up between PRP and CS groups. A network meta-analysis by Babatunde et al.[5] for the comparative effectiveness of different treatment options, including CS injection, has shown an equivocal result. Cochrane database of systematic reviews on the treatment of plantar heel pain[8] found low-quality evidence that when local CS injection was compared with placebo or no treatment, heel pain was reduced slightly up to one month but not beyond that. A similar observation was made by Karls et al.[36]
Rupture of plantar fascia following repeated CS injections is rare but a known complication. In a retrospective study of 120 PF patients, 2.4% of cases reported plantar fascia rupture. following an average of 2.67 injections.[37] Suzue et al.[38] reported a case of plantar fascia rupture after 2nd CS injection in a young professional soccer player. Although a single dose of CS injection was given in Group B patients, clinical evaluation was done at each follow-up to rule out plantar fascia rupture Complications such as calcaneal osteomyelitis and heel abscess have been reported by Mohd Khalid and Bajuri following CS injection in an elderly lady with diabetes and rheumatoid arthritis.[39] PF patients with associated comorbidities such as uncontrolled diabetes and rheumatoid arthritis were excluded from the current study. Hence, we did not encounter any such complications in our study groups.
Besides PRP and CS, other treatment modalities for PF such as NSAID, physical therapy, ultrasound therapy, autologous whole blood, ESWT, dry needling, and Botulinum toxin cannot be ignored. The network meta-analysis by Haibo Li et al.[40] on comparing the efficacy of eight treatment modalities for PF revealed that ESWT ranked the first and was considered the most optimal treatment. In contrast, Botulinum toxin A and PRP remain suboptimal treatment. Currently, Raeissadat et al.[41] have used high-molecular-weight Hyaluronic acid and shown good results as CS. Despite extensive research on various modalities of treatment for PF, observations are continuing to be controversial. Further research with larger sample size is required on its basic pathophysiology to specify the ideal management methods.
Limitations
Blinding was not done during the allocation of samples; hence, the possibility of selection bias cannot be ruled out. The use of simple randomization in a sample size less than a hundred is a limitation to this study. The study duration was limited to 6 months, which may not be sufficient to study the long-term impacts. Compliance with the home rehabilitation program and its impact on results is not measured. Before administering PRP injection, whether the platelets should be activated or not, is not well established. Scherer et al.[42] observed in their animal model that nonactivated platelets stimulated wound healing more efficiently than activated platelets. Platelets, once activated with thrombin or calcium, release most of their growth factors and loses their ability, specifically to interact with the environment.
Conclusion
CS has an early effect, reducing pain to a moderate level (82.4%) in comparison to PRP (P = 0.000). However, the effect is not sustainable over a long period. On the other hand, PRP was found to have better pain relief over 3 months and 6 months follow-up with a mean VAS score of 2.0 ± 0.9 and 0.8 ± 0.8, respectively (P = 0.000). There is a significant improvement in foot function and patient satisfaction as well at 6 months follow up (P = 0.000). Therefore, PRP can be advised for a sustained and prolong impact on chronic PF.
Recommendations
Further studies are needed to find out the results of platelet activation before PRP injection. Whether single or multiple doses of PRP injection are required to achieve the desired result, needs to be established.
Ethical consideration
With the patient's informed consent, the study has been done in accordance with the Declaration of Helsinki. The institutional review board approved the study in December 2017. Ethical committee of Swami Vivekananda National Institute of Rehabilitation Training and Research held on 18/12/2017.
Acknowledgment
We would like to thank Mr B B Nanda for his expert technical assistance in statistical analysis.
Financial support and sponsorship
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
There are no conflicts of interest.
Author's contribution
PKS designed and supervised the study, interpreted the results of the study, revised the manuscript for critical intellectual content, and agrees to be accountable for all aspects of the work and to take the responsibility of the corresponding author. NAU collected the data, statistically analyzed the data, and prepared an initial draft of the manuscript. SPD revised the manuscript critically by providing important intellectual inputs. All authors have critically reviewed and approved the final version of the manuscript and are responsible for the manuscript's content and similarity index.
| 1. | Riel H, Cotchett M, Delahunt E, Rathleff MS, Vicenzino B, Weir A, et al. Is 'plantar heel pain' a more appropriate term than 'plantar fasciitis'? Time to move on. Br J Sports Med 2017;51:1576-7. [Google Scholar] |
| 2. | Rasenberg N, Bierma-Zeinstra SM, Bindels PJ, van der Lei J, van Middelkoop M. Incidence, prevalence, and management of plantar heel pain: A retrospective cohort study in Dutch primary care. Br J Gen Pract 2019;69:e801-8. [Google Scholar] |
| 3. | Scher DL, Belmont PJ Jr, Bear R, Mountcastle SB, Orr JD, Owens BD. The incidence of plantar fasciitis in the United States military. J Bone Joint Surg Am 2009;91:2867-72. [Google Scholar] |
| 4. | Hill CL, Gill TK, Menz HB, Taylor AW. Prevalence and correlates of foot pain in a population-based study: The North West Adelaide health study. J Foot Ankle Res 2008;1:2. [Google Scholar] |
| 5. | Babatunde OO, Legha A, Littlewood C, Chesterton LS, Thomas MJ, Menz HB, et al. Comparative effectiveness of treatment options for plantar heel pain: A systematic review with network meta-analysis. Br J Sports Med 2019;53:182-94. [Google Scholar] |
| 6. | Uǧurlar M, Sönmez MM, Uǧurlar ÖY, Adıyeke L, Yıldırım H, Eren OT. Effectiveness of four different treatment modalities in the treatment of chronic plantar fasciitis during a 36-month follow-up period: A randomized controlled trial. J Foot Ankle Surg Off Publ Am Coll Foot Ankle Surg 2018;57:913-8. [Google Scholar] |
| 7. | Whittaker GA, Munteanu SE, Menz HB, Bonanno DR, Gerrard JM, Landorf KB. Corticosteroid injection for plantar heel pain: A systematic review and meta-analysis. BMC Musculoskelet Disord 2019;20:378. [Google Scholar] |
| 8. | David JA, Sankarapandian V, Christopher PR, Chatterjee A, Macaden AS. Injected corticosteroids for treating plantar heel pain in adults. Cochrane Database Syst Rev 2017;6:CD009348. [Google Scholar] |
| 9. | Singh P, Madanipour S, Bhamra JS, Gill I. A systematic review and meta-analysis of platelet-rich plasma versus corticosteroid injections for plantar fasciopathy. Int Orthop 2017;41:1169-81. [Google Scholar] |
| 10. | Hsiao MY, Hung CY, Chang KV, Chien KL, Tu YK, Wang TG. Comparative effectiveness of autologous blood-derived products, shock-wave therapy and corticosteroids for treatment of plantar fasciitis: A network meta-analysis. Rheumatology (Oxford) 2015;54:1735-43. [Google Scholar] |
| 11. | Filardo G, Kon E, Di Matteo B, D Martino A, Tesei G, Pelotti P, et al. Platelet-rich plasma injections for the treatment of refractory achilles tendinopathy: Results at 4 years. Blood Transfus 2014;12:533-40. [Google Scholar] |
| 12. | Descalzi F, Ulivi V, Cancedda R, Piscitelli F, Luongo L, Guida F, et al. Platelet-rich plasma exerts antinociceptive activity by a peripheral endocannabinoid-related mechanism. Tissue Eng Part A 2013;19:2120-9. [Google Scholar] |
| 13. | Soraganvi P, Nagakiran KV, Raghavendra-Raju RP, Anilkumar D, Wooly S, Basti BD, et al. Is platelet-rich plasma injection more effective than steroid injection in the treatment of chronic plantar fasciitis in achieving long-term relief? Malays Orthop J 2019;13:8-14. [Google Scholar] |
| 14. | Raeissadat SA, Nouri F, Darvish M, Esmaily H, Ghazihosseini P. Ultrasound-guided injection of high molecular weight hyaluronic acid versus corticosteroid in management of plantar fasciitis: A 24-week randomized clinical trial. J Pain Res 2020;13:109-21. [Google Scholar] |
| 15. | Martinelli N, Marinozzi A, Carnì S, Trovato U, Bianchi A, Denaro V. Platelet-rich plasma injections for chronic plantar fasciitis. Int Orthop 2013;37:839-42. [Google Scholar] |
| 16. | Sun K, Zhou H, Jiang W. Extracorporeal shock wave therapy versus other therapeutic methods for chronic plantar fasciitis. Foot Ankle Surg 2020;26:33-8. [Google Scholar] |
| 17. | Vahdatpour B, Kianimehr L, Moradi A, Haghighat S. Beneficial effects of platelet-rich plasma on improvement of pain severity and physical disability in patients with plantar fasciitis: A randomized trial. Adv Biomed Res 2016;5:179. [Google Scholar] |
| 18. | Budiman-Mak E, Conrad KJ, Roach KE. The foot function index: A measure of foot pain and disability. J Clin Epidemiol 1991;44:561-70. [Google Scholar] |
| 19. | Physical Medicine and Rehabilitation 4th edition. Available from: https://www.elsevier.com/books/physical-medicine-and-rehabilitation/braddom/978-1-4377-0884-4. [Last accessed on 2020 May 14]. [Google Scholar] |
| 20. | Digiovanni BF, Nawoczenski DA, Malay DP, Graci PA, Williams TT, Wilding GE, et al. Plantar fascia-specific stretching exercise improves outcomes in patients with chronic plantar fasciitis. A prospective clinical trial with two-year follow-up. J Bone Joint Surg Am 2006;88:1775-81. [Google Scholar] |
| 21. | Shetty SH, Dhond A, Arora M, Deore S. Platelet-rich plasma has better long-term results than corticosteroids or placebo for chronic plantar fasciitis: Randomized control trial. J Foot Ankle Surg 2019;58:42-6. [Google Scholar] |
| 22. | Peerbooms JC, Lodder P, den Oudsten BL, Doorgeest K, Schuller HM, Gosens T. Positive effect of platelet-rich plasma on pain in plantar fasciitis: A double-blind multicenter randomized controlled trial. Am J Sports Med 2019;47:3238-46. [Google Scholar] |
| 23. | Mahindra P, Yamin M, Selhi HS, Singla S, Soni A. Chronic plantar fasciitis: Effect of platelet-rich plasma, corticosteroid, and placebo. Orthopedics 2016;39:e285-9. [Google Scholar] |
| 24. | Donald MJ, Derbyshire S. Lignocaine toxicity; a complication of local anaesthesia administered in the community. Emerg Med J 2004;21:249-50. [Google Scholar] |
| 25. | Bausset O, Magalon J, Giraudo L, Louis ML, Serratrice N, Frere C, et al. Impact of local anaesthetics and needle calibres used for painless PRP injections on platelet functionality. Muscles Ligaments Tendons J 2014;4:18-23. [Google Scholar] |
| 26. | Carofino B, Chowaniec DM, McCarthy MB, Bradley JP, Delaronde S, Beitzel K, et al. Corticosteroids and local anesthetics decrease positive effects of platelet-rich plasma: AnIn vitro study on human tendon cells. Arthroscopy 2012;28:711-9. [Google Scholar] |
| 27. | Yang WY, Han YH, Cao XW, Pan JK, Zeng LF, Lin JT, et al. Platelet-rich plasma as a treatment for plantar fasciitis: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e8475. [Google Scholar] |
| 28. | Barman A, Mukherjee S, Sahoo J, Maiti R, Rao PB, Sihna M K, et al. Single intra-articular platelet-rich plasma versus corticosteroid injections in the treatment of adhesive capsulitis of the shoulder: A cohort study. Am J Phys Med Rehabil 2019;98:549-57. [Google Scholar] |
| 29. | Lee H-R, Park KM, Joung YK, Park KD, Do SH. Platelet-rich plasma loaded hydrogel scaffold enhances chondrogenic differentiation and maturation with up-regulation of CB1 and CB2. J Control Release Off J Control Release Soc 2012;159:332-7. [Google Scholar] |
| 30. | Chen YJ, Wu YC, Tu YK, Cheng JW, Tsai WC, Yu TY. Autologous blood-derived products compared with corticosteroids for treatment of plantar fasciopathy: A systematic review and meta-analysis. Am J Phys Med Rehabil 2019;98:343-52. [Google Scholar] |
| 31. | Jain K, Murphy PN, Clough TM. Platelet rich plasma versus corticosteroid injection for plantar fasciitis: A comparative study. Foot (Edinb) 2015;25:235-7. [Google Scholar] |
| 32. | Monto RR. Platelet-rich plasma and plantar fasciitis. Sports Med Arthrosc Rev 2013;21:220-4. [Google Scholar] |
| 33. | Monto RR. Platelet-rich plasma efficacy versus corticosteroid injection treatment for chronic severe plantar fasciitis. Foot Ankle Int 2014;35:313-8. [Google Scholar] |
| 34. | Ling Y, Wang S. Effects of platelet-rich plasma in the treatment of plantar fasciitis: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2018;97:e12110. [Google Scholar] |
| 35. | Jain SK, Suprashant K, Kumar S, Yadav A, Kearns SR. Comparison of plantar fasciitis injected with platelet-rich plasma vs. corticosteroids. Foot Ankle Int 2018;39:780-6. [Google Scholar] |
| 36. | Karls SL, Snyder KR, Neibert PJ. Effectiveness of corticosteroid injections in the treatment of plantar fasciitis. J Sport Rehabil 2016;25:202-7. [Google Scholar] |
| 37. | Kim C, Cashdollar MR, Mendicino RW, Catanzariti AR, Fuge L. Incidence of plantar fascia ruptures following corticosteroid injection. Foot Ankle Spec 2010;3:335-7. [Google Scholar] |
| 38. | Suzue N, Iwame T, Kato K, Takao S, Tateishi T, Takeda Y, et al. Plantar fascia rupture in a professional soccer player. J Med Investig JMI 2014;61:413-6. [Google Scholar] |
| 39. | Mohd Khalid S, Bajuri M. Unexpected sequelae of plantar fasciitis: Iatrogenic calcaneal osteomyelitis following plantar heel injection. Malays Fam Physician Off J Acad Fam Physicians Malays 2019;14:80-3. [Google Scholar] |
| 40. | Li H, Lv H, Lin T. Comparison of efficacy of eight treatments for plantar fasciitis: A network meta-analysis. J Cell Physiol 2018;234:860-70. [Google Scholar] |
| 41. | Ultrasound-Guided Injection of High Molecular Weight Hyaluronic Acid versus Corticosteroid in Management of Plantar Fasciitis: A 24-Week Randomized Clinical Trial. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6969680/. [Last accessed on 2020 Mar 26]. [Google Scholar] |
| 42. | Scherer SS, Tobalem M, Vigato E, Heit Y, Modarressi A, Hinz B, et al. Nonactivated versus thrombin-activated platelets on wound healing and fibroblast-to-myofibroblast differentiationin vivo and in vitro. Plast Reconstr Surg 2012;129:46e-54e. [Google Scholar] |
Fulltext Views
3,697
PDF downloads
429





