Translate this page into:
Legg-Calve-Perthes disease: Impact of prognostic criteria on management plan
Corresponding Author:
Mohamed M Zamzam
Department of Orthopaedics, College of Medicine, King Saud University, P O Box 7805, Riyadh 11472
Saudi Arabia
mzamzam@ksu.edu.sa
| How to cite this article: Zamzam MM. Legg-Calve-Perthes disease: Impact of prognostic criteria on management plan. J Musculoskelet Surg Res 2021;5:1-3 |
Legg-Calve-Perthes disease (LCPD) is an idiopathic self-limited condition resulting from interruption of the blood supply of the femoral head in children.[1],[2],[3] Boys are affected more than girls (4:1 ratio), with a mean age of onset of 6 years, ranging from 2 years to skeletal maturity.[1],[4],[5] The incidence of LCPD varies between 0.2 and 19 per 100,000 of the pediatric population. Suggested risk factors are race, repetitive trauma, anomalies of the blood supply, coagulopathy, and obesity.[2],[4],[6]
The disruption of femoral head blood supply leads to changes in the acetabulum, femoral head, and its growth plate. During subsequent revascularization, the femoral head becomes soft and vulnerable to deformity by weight loading. This may result in a change in shape, flattening, or even subluxation. Consequently, a broad spectrum of pathological events occurs from mild forms without sequelae to severe deformities predisposing to early osteoarthritis of the hip joint.[1],[4],[7],[8]
The classical clinical presentation of LCPD is limping and localized thigh and/or knee pain, usually after physical activity, in an otherwise healthy child. Typically, there are limitations in the abduction and internal rotation of the involved hip, and Trendelenburg gait, which is pathognomonic during advanced stages of the disease.[1],[4]
The disease course includes four stages. The stage of necrosis is the initial one that usually lasts for around 6 months. Fragmentation is the next stage, in which revascularization occurs and removes the necrotic bone that looks fragmented in the radiographs as decreased bone density and lasts for another 6 months. During fragmentation, subchondral fracture, femoral head collapse, and lateralization may occur. The third stage starts with reossification and may continue for more than 3 years. The femoral head's growth disturbance may occur in the final stage leading to femoral neck widening and shortening, greater trochanter overgrowth, and limb length discrepancy.[9]
The plain radiograph is the most useful imaging modality to assess the LCPD stage, the extent of femoral head involvement and its containment in the acetabulum.[1] Other imaging modalities, such as magnetic resonance imaging and pneumoarthrography, can provide more comprehensive information in an early stage of the disease and evaluate cases at risk.[3],[10]
To predict the outcome and decide on the suitable management, a classification with prognostic characteristics should be helpful. The overall prognosis depends on the femoral head congruity with the acetabulum at maturity that will directly influence the occurrence of osteoarthritis. Clinical prognostic factors include age, gender, weight, and hip motion. Radiological prognostic factors include the three well-known classifications that mostly take the position and extent of femoral head involvement into account. Interobserver reliability ranges from poor to fair for the Salter–Thompson classification, fair to moderate for the Catterall classification, and moderate to good for the Herring (lateral pillar) classification.[1],[6] Radiological factors also include the presence of signs of “hip-at-risk” and reduction in abduction in the plain radiograph.[1] Recently, there is consensus among pediatric orthopedic surgeons that the most reliable prognostic factors for LCPD are the age of onset and presence of lateralization.
A favorable outcome is expected in 85% of patients. The outcome is almost always unfavorable in the very severe cases of LCPD. Femoral head deformity alone is not considered a poor prognostic sign. The Stulberg classification remains the standard reference for the assessment of prognosis and outcome at skeletal maturity.[11] The classification predicts the prognosis by observing the femoral head's deformity and congruity in relation to the acetabulum. Nevertheless, Stulberg classification does not help to decide on the treatment modality. The sphericity deviation score (SDS) is promising, but not yet in routine use, as its relations to long-term outcome remain unconfirmed. Although SDS has good interobserver and intraobserver reproducibility,[12] the lateral pillar involvement is considered a significant predictor for trochanteric overgrowth and limb length discrepancy.[13]
The benchmarks for LCPD definitive treatment have not been established because its exact etiology and pathology have not been completely clarified.[11] The aim of treating LCPD is to reduce the risk of future hip osteoarthritis by preventing deformity of the femoral head, which may occur with loss of reasonable containment. Containment should be attained during the fragmentation and reossification phase to allow the acetabulum to act as a mold for the femoral head during the healing and revascularization phase.[8],[14]
Most pediatric orthopedic surgeons agree now on the concept of femoral head containment within the acetabulum during the treatment of LCPD.[3],[5],[15] Loss of containment at any stage of the disease is an important signal to start adequate conservative or surgical treatment as soon as possible to prevent unfavorable outcomes.[3],[6],[16] Every child must get an accustomed treatment specific for him/her and the follow-up should continue at regular intervals till skeletal maturity.
Nonoperative measures to obtain containment include abduction casts or bracing, but these orthoses require prolonged use and are usually difficult to tolerate by the child and family.[4] Proposed surgical methods to obtain containment include proximal femoral varus osteotomy, pelvic osteotomy, and shelf acetabuloplasty. Pelvic osteotomy, combined with a proximal femoral varus osteotomy, can provide better containment in children with more severe deformity.[7],[17] Hinge abduction is considered a contraindication to redirectional pelvic or femoral varus osteotomy to achieve containment. However, good results have been reported after shelf acetabuloplasty or Chiari osteotomy.[18] Femoroacetabular impingement should be considered if there is a limitation of motion or pain with extreme movement, and thus proper physical therapy should commence.[4]
Children <6 years of age often have a good prognosis [Figure - 1]. Herring et al., in a prospective multicenter study, concluded that age at the onset of the disease and the lateral pillar classification are strongly linked with the final outcome in children with LCPD. These two factors are useful to decide on nonoperative versus operative management.[9] Children of more than 8 years of age with lateralized femoral head should be addressed to obtain containment [Figure - 2]. Once diagnosed, surgery to achieve containment should be performed in the fragmentation stage.[4]
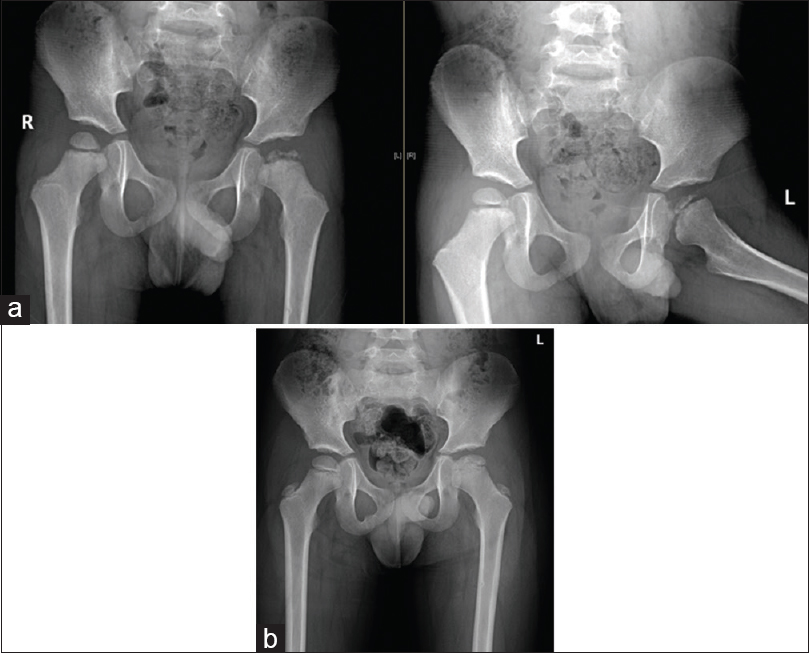 |
| Figure 1: (a) Anterior–posterior and abduction-external rotation views of the left hip with Legg-Calve-Perthes disease in a 3½-year-old boy with total femoral head involvement. As an early-onset Legg-Calve-Perthes disease with good containment, surgical intervention was not required during follow-up. (b) Anterior–posterior standing view of both hips, showing an excellent outcome after 3 years |
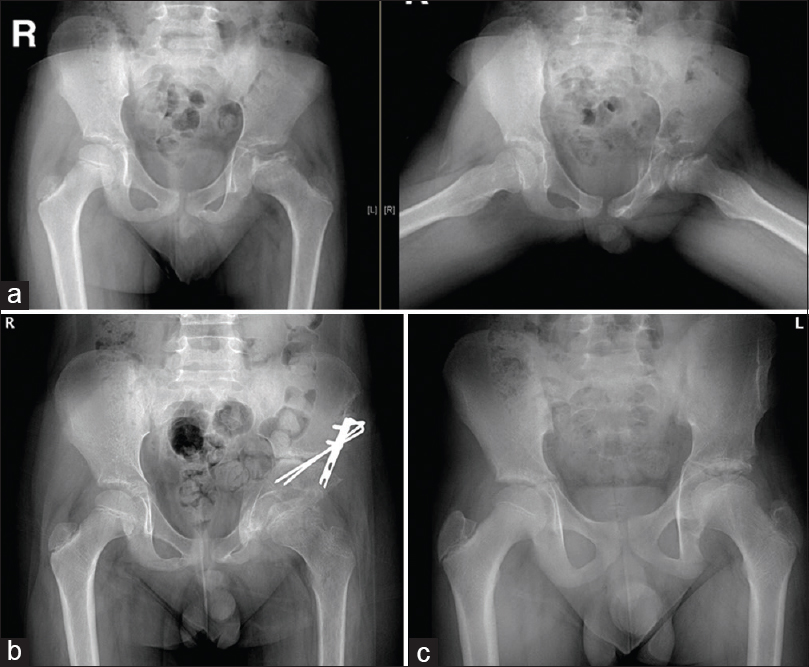 |
| Figure 2: (a) Anterior–posterior and abduction-external rotation views of the left hip with Legg-Calve-Perthes Disease in an 11-year-old boy with femoral head lateralization, which could be reduced in abduction. (b) Anterior–posterior view of both hips, showing good containment of the left hip after triple innominate osteotomy. (c) Anterior–posterior standing view of both hips, showing excellent results 3 years after surgical containment procedure |
Arthrodiastasis of the hip joint with soft-tissue release is considered when other treatment options are contraindicated, as it could be done in cases with hip stiffness or deformities. It is supposed to improve the range of motion and reduce lateralization.[19] Proximal femoral valgus osteotomy can be performed to reduce the hinged abduction and improve the range of motion.[20] Transtrochanteric rotational osteotomy is a new salvage procedure for patients with late-onset LCPD.[21] In several recent studies, promising results were attained with bisphosphonate therapy. These data open up the possibility of pharmacological treatments for LCPD that may be developed in future.[6]
In conclusion, the role of treating LCPD is to end the course of the disease with a spherical femoral head as much as possible that is congruent with the acetabulum to avoid early degenerative changes. LCPD with early onset is expected to have a favorable outcome and rarely requires surgical intervention. The extent of femoral head involvement alone is not an indication for operative treatment. Surgery should be reserved for children of older age with loss of femoral head containment.
Declaration of patient consent
The authors certify that they have obtained all appropriate parents of the patients consent forms. In the form the parents have given their consents for the images and other clinical information to be reported in the journal. The parents understand that the names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
| 1. | Rampal V, Clément JL, Solla F. Legg-Calvé-Perthes disease: Classifications and prognostic factors. Clin Cases Miner Bone Metab 2017;14:74-82. [Google Scholar] |
| 2. | Hailer YD, Hailer NP. Is Legg-Calvé-Perthes disease a local manifestation of a systemic condition? Clin Orthop Relat Res 2018;476:1055-64. [Google Scholar] |
| 3. | Jandl NM, Schmidt T, Schulz M, Rüther W, Stuecker MH. MRI and sonography in Legg-Calvé-Perthes disease: Clinical relevance of containment and influence on treatment. J Child Orthop 2018;12:472-9. [Google Scholar] |
| 4. | Mazloumi SM, Ebrahimzadeh MH, Kachooei AR. Evolution in diagnosis and treatment of Legg-Calve-Perthes disease. Arch Bone Jt Surg 2014;2:86-92. [Google Scholar] |
| 5. | Kadhim M, Holmes L Jr, Bowen JR. The role of shelf acetabuloplasty in early and late stages of Perthes disease: A meta-analysis of observational studies. J Child Orthop 2012;6:379-90. [Google Scholar] |
| 6. | Leroux J, Abu Amara S, Lechevallier J. Legg-Calvé-Perthes disease. Orthop Traumatol Surg Res 2018;104:S107-12. [Google Scholar] |
| 7. | Hsu JE, Baldwin KD, Tannast M, Hosalkar H. What is the evidence supporting the prevention of osteoarthritis and improved femoral coverage after shelf procedure for Legg-Calvé-Perthes disease? Clin Orthop Relat Res 2012;470:2421-30. [Google Scholar] |
| 8. | Elzohairy MM. Short follow-up evaluation of proximal femoral varus osteotomy for treatment of Legg-Calvé-Perthes disease. J Orthop Traumatol 2016;17:345-51. [Google Scholar] |
| 9. | Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part I: Classification of radiographs with use of the modified lateral pillar and Stulberg classifications. J Bone Joint Surg Am 2004;86:2103-20. [Google Scholar] |
| 10. | Milani C, Dobashi ET. Arthrogram in Legg-Calvé-Perthes disease. J Pediatr Orthop 2011;31:S156-62. [Google Scholar] |
| 11. | Stulberg SD, Cooperman DR, Wallensten R. The natural history of Legg-Calvé-Perthes disease. J Bone Joint Surg Am 1981;63:1095-108. [Google Scholar] |
| 12. | Siddesh ND, Shah H, Tercier S, Pai H, Nair S, Joseph B. The sphericity deviation score: A quantitative radiologic outcome measure of Legg-Calvé Perthes disease applicable at the stage of healing and at skeletal maturity. J Pediatr Orthop 2014;34:522-8. [Google Scholar] |
| 13. | Herring JA, Kim HT, Browne R. Legg-Calve-Perthes disease. Part II: Prospective multicenter study of the effect of treatment on outcome. J Bone Joint Surg Am 2004;86:2121-34. [Google Scholar] |
| 14. | Joseph B, Price CT. Principles of containment treatment aimed at preventing femoral head deformation in Perthes disease. Orthop Clin North Am 2011;42:317-27. [Google Scholar] |
| 15. | Kamegaya M, Morita M, Saisu T, Kakizaki J, Oikawa Y, Segawa Y. Single versus combined procedures for severely involved Legg-Calvé-Perthes disease. J Pediatr Orthop 2018;38:312-9. [Google Scholar] |
| 16. | Manig M. Legg-Calvé-Perthes disease (LCPD). Principles of diagnosis and treatment. Orthopade 2013;42:891-902. [Google Scholar] |
| 17. | Lim KS, Shim JS. Outcomes of combined shelf acetabuloplasty with femoral varus osteotomy in severe Legg-Calve-Perthes (LCP) disease: Advanced containment method for severe LCP disease. Clin Orthop Surg 2015;7:497-504. [Google Scholar] |
| 18. | Reinker KA. Shelf and/or reduction and containment surgery. Orthop Clin North Am 2011;42:355-9. [Google Scholar] |
| 19. | Kim SS, Lee CW, Kim HJ, Kim HH, Wang L. Treatment of late-onset Legg-Calve-Perthes disease by arthrodiastasis. Clin Orthop Surg 2016;8:452-7. [Google Scholar] |
| 20. | Yoo WJ, Choi IH, Moon HJ, Chang S, Cho TJ, Choi YH, et al. Valgus femoral osteotomy for noncontainable Perthes hips: Prognostic factors of remodeling. J Pediatr Orthop 2013;33:650-5. [Google Scholar] |
| 21. | Nakashima Y, Kubota H, Yamamoto T, Mawatari T, Motomura G, Iwamoto Y. Transtrochanteric rotational osteotomy for late-onset Legg-Calve-Perthes disease. J Pediatr Orthop 2011;31:S223-8. [Google Scholar] |
Fulltext Views
3,877
PDF downloads
1,633





