Translate this page into:
Managing the nerve gap: New tools in the peripheral nerve repair toolbox
Corresponding Author:
Mohammad Nassimizadeh
The Peripheral Nerve Injury Service, Queen Elizabeth Hospital, Birmingham
UK
a.nassimizadeh@gmail.com
| How to cite this article: Nassimizadeh M, Nassimizadeh AK, Power D. Managing the nerve gap: New tools in the peripheral nerve repair toolbox. J Musculoskelet Surg Res 2019;3:4-8 |
Abstract
End-to-end repair of a peripheral nerve transection injury remains the gold standard. Delayed repair, nerve debridement and early functional mobilisation may all increase repair site tension, which impedes axon regeneration and must be avoided. Prompt diagnosis, referral to a specialist and exploration can minimise the nerve retraction, debridement and gap size, and societal benefit will be achieved through adopting a standardised approach to management. However, early exploration may provide challenges in defining the extent of the injury zone and therefore the adequacy of nerve debridement. Repair site tension can be reduced with 'sutureless' nerve approximation in a conduit, interposition of autologous graft or with interposed processed nerve allograft. Sutures can be avoided through interposition de-tensioning grafts and use of tissue glues. However, a large gap in a conduit will not support robust regeneration and grafts have two neurorrhaphy sites for axons to negotiate. Autologous graft has a donor site morbidity that may be unacceptable. An algorithm for peripheral nerve reconstruction should include the use of conduits and allograft as de-tensioning devices, avoiding the morbidity associated with autologous nerve grafting.Introduction
Nerve discontinuity injuries follow trauma, oncological resection or as a result of inadvertent injury during medical procedures. The consequent loss of function is related to the site of injury, the function of the injured nerve and the type of nerve fibre sub-types contained therein. A mixed motor-sensory nerve injury will be associated with sensory loss, pain and paralysis.[1] Factors contributing to the outcome following repair or reconstruction of an injured nerve include the nerve type, severity of injury, anatomical site, delay between injury and reconstruction, method of repair, quality of the repair, surgical bed at the repair site, tension at the repair site, re-innervation distance, end-organ integrity, age of the patient and comorbidities.[2] Early diagnosis and reconstruction will greatly influence outcomes on a health economic scale. Some factors cannot be controlled. The technical factors that may be influenced include the repair method, quality and tension across the repair site.
Nerve gap occurs following transection due to the loss of biotensegrity.[3],[4] Biotensegrity is the concept that all biological tissues have longitudinal fibres that are normally pre-tensioned. A transection of these structures and hence longitudinal fibres cause fibres to lose their pre-tensioning, and hence, the cut ends retract. Delay to repair results in increased modulus of elasticity in the nerve and greater tension forces on closing the gap, partly because the pre-tensioning has been restored. Delay also is associated with scar formation at the cut nerve ends that will hamper neural regeneration. The nerve ends must be debrided, increasing the injury gap to be reconstructed. Direct suturing of a nerve will create tension at the repair site and strain concentration is at the suture–nerve interface, which may cause ischaemia, inflammation and fibrosis. Suture failure under loading widens this potential zone of injury and scar and may result in a neuroma-in-continuity at the repair site. Without delay or nerve tissue loss, there remains a stress concentration at the repair site due to loss of the nerve integrity. Providing a favourable environment for axon regeneration is a key to minimise intra-neural scar.
Direct end-to-end tension-free repair of an injured nerve remains the gold standard, but the inconsistent clinical results are drivers for change. Regenerating axons slow and have difficulty transversing a disorganised intra-neural architecture or scar tissue within the zone of trauma and repair.[2],[5],[6] Optimisation of regeneration requires debridement of damaged nerve, maintaining alignment of fascicles and reducing scar formation at the repair site. When the nerve gap is too large to repair directly without tension, interposition of a nerve graft may minimise tension; however, regenerating axons must successfully negotiate two coaptation sites.[7],[8],[9]
Alternative strategies include the use of conduits and processed nerve allograft as de-tensioning devices and avoiding donor autologous autograft harvest morbidity.
Nerve Conduits
Nerve conduits provide a favourable microenvironment that allows the two ends of the nerve to be placed within a tube that bridges the gap, allowing axonal regeneration towards the distal nerve. The conduit prevents extrinsic scar invading the repair site and intrinsic scar formation between the nerve ends, by creating a physical barrier isolating the repair. Conduits are designed to bridge small nerve gaps. Fibrin clot formation within the conduit between the nerve ends creates the scaffold to support Schwann cell and axonal migration[10],[11] within a microenvironment that is enriched with neurotrophic factors.[12],[13] Unsupported growth can reliably cross gaps of a few millimetres; however, evidence to support use in gaps beyond 12 mm is limited.
Conduits may be used in nerve repair without tissue loss to create an effective lengthening of the nerve, thereby reducing tension. Sutures are placed remotely between the ends of the conduit and the adjacent epineurium, creating a de-tensioned segment between the suture lines where longitudinal strain forces are concentrated. The benefit of avoiding sutures at the repair site must be weighed against the potential barrier to regeneration of creating a small gap at the repair site.
An alternate strategy is in a nerve that may be repaired directly without tissue loss; a conduit sited across the repair site can be used to create a detensioned suture site through placing remote sutures between the conduit ends and the adjacent epineurium. The evidence to support this use of conduits is currently limited, but it remains an attractive solution through supporting the repair site, preventing scar ingress and maintaining an optimised microenvironment with concentration of neurotrophic factors at the repair site.
The benefits of a conduit-assisted repair in a primary nerve repair include de-tensioning of the repair site and minimising repair site sutures. The benefits in small gap management include avoiding an autologous graft donor deficit, reduced operating time, avoidance of two suture lines and proven clinical effectiveness. The conduit approximation may also be technically simpler to achieve an adequate quality of repair compared with microsurgical neurorrhaphy. A study of intentionally misaligned sciatic nerves repairs in a rodent model demonstrated better recovery with a conduit repair than a direct repair.[14]
Non-absorbable silicone conduits demonstrated effectiveness for nerve regeneration but eventually produced an inflammatory response and irritation at the repair site necessitating a second operation for removal.[15],[16] Contemporary conduits are available in bioresorbable polymers of polycaprolactone (Neurolac® Polyganics, Netherlands) and polyglycolic acid,[17] collagen (Neuragen® Integra)[18] and chitosan (Reaxon).[19],[20] They are semi-permeable, allowing nutrient and oxygen diffusion; semi-rigid, preventing occlusion from distortion or collapse during motion and either reabsorb or integrate, reducing the risk of irritation and need for removal. The Neurolac and Reaxon conduits are transparent, permitting an operating surgeon to see the alignment of the nerve ends at the repair site.
The AxoGuard® Nerve Connector (AxoGen Inc., Alachua, Florida, USA) is new development in the field of conduit that is designed as a coaptation aided for situations where there is no nerve tissue loss. The device is more flexible than traditional conduits and is not compression resistant. It is contra-indicated in gap management unless used in conjunction with processed nerve allograft. The AxoGuard® is designed for sutureless neurorrhaphy with remote sutures or for de-tensioning of a suture repair site.
One of the key concerns when considering a conduit repair is nerve diameter and gap size. Gap size has been heavily published with strong evidence for short repair up to 3 cm, but reports have been published of using conduits in longer gaps. Lundborg et al. reported equivalent recovery using silicone conduits in 5 mm ulnar and median nerve gaps versus direct repair.[16] Taras et al. reported good recovery looking at 22 digital nerve repairs with a mean gap of 12 mm while Rinker and Liau reported 36 digital nerves repaired with conduits with a gap of 9 mm.[21],[22] Weber et al. looked at a prospective review of digital nerve repairs with conduits compared to autograft. They concluded that for 3 cm defects, conduits produced better recovery but recovery decreased the longer the gap.[23]
One of the limiting factors with conduits is nerve size. While efficacy has been demonstrated in smaller nerves, much smaller gaps show success in larger nerves. The primary cause for this is related to fibrin clot instability, which has been shown by Lundborg et al.[24] This unstable fibrin clot limits axonal regeneration along the conduit.
Conduits have been shown to be effective in short gaps and as previously discussed tension-free repair is the gold standard. This combination of factors leads to the potential use in direct nerve repairs with no nerve loss. Whenever a nerve is sectioned, it inherently retracts. In primary repairs, these ends can be pulled to oppose each other, which create a level of tension at the repair site. The question here would be whether conduits can be used in primary repairs to avoid this tension and avoid sutures at the repair site leading to more favourable outcomes. This is particularly an important question in injured nerves that retract over time if an immediate repair is not possible. This question is being addressed by the connect trial that is using conduits to de-tension repairs RCT.
Allograft
Many nerve injuries involve larger nerves. Due to conduits limits in larger nerve defects and larger nerve sizes due to the fibrin instability, the surgeon can benefit from another tool to help in these deficits. What is needed in these larger defects is a stable architecture to allow axonal regeneration. This can be found in nerve allograft.
These allografts offer the same benefits of conduits with no donor deficit, clinically proven effectiveness, reduced operating time (no harvesting) and an unlimited supply unlike autografts, provided there are financial funds. Previous limits on allograft included the immunogenicity of nerve tissue needing immunosuppression.[25] Immunosuppression has inherent toxicity that is systemic.
However, advances in tissue processing with detergents and processing can lead to de-cellularising of immunogenic components from nerve allografts. This, unfortunately, leads to the loss of Schwann cells but does leave a cellular architecture that aids axonal regeneration.[26],[27] This structure combined with neurotrophic factors within the allograft provides a good option for larger defects.
At present, there are a few allografts on the shelf produced by AxoGen as well as fresh options, and there is a processed option in China. The Axogen process aims to remove cellular components while maintaining the structure that is crucial for nerve recovery. This is achieved through detergent washes and enzyme treatments to remove neurotoxic glycoprotein from the basement membrane followed by gamma irradiation and frozen challenges HTA to remove cellular components.
When comparing the mid-graft axonal density, histology showed similarity in allograft and isograft.[28] Within humans, researchers at the Mayo Clinic looked at upper limb sensory nerve gaps of 0.5–3 cm and showed good results with patient recovery with a 6 mm two-point discrimination or better.[29]
Allografts are kept frozen until they are needed and the nerve gap is identified. The biggest drawback is that just like an autograft, there are two points of end-to-end anastomosis. The allograft has a denser composition that makes handling easier, but skill is still needed to pick the correct size of allograft. The reconstruction of larger nerves can also provide a denser architecture than aligning several sural grafts (cable graft) with tissue glue, without the limitation of length. The maximum diameter is limited to 4–5 mm due to poor revascularisation of larger grafts.
Although Schwann cell migration limits length of allograft repair, the exact length is not very clear. A group of 20 patients with 30–50 mm defects showed 90% rate of recovery (S3–4 and M3–5).[30]
One factor that limits autograft is the central necrosis of tissue due to the thickness exceeding that of nutrient and oxygen diffusion. This can lead to intra-neural scarring and decrease axonal density in the recovering nerve. Since allograft does not contain living tissue, this is not a factor. However, axonal regeneration and Schwann cells have a metabolic demand that needs to be met by neovascularisation or diffusion. Tang et al. tried to address this by comparing cable graft versus allograft in rodents; however, even the thickest allograft is below the size that would be limited by diffusion or nutrients.[31]
Allograft and Wrap?
Coaptation-assisted repair of a nerve repair is a new concept and aims to unify the benefits of both conduits and allografts. While allografts remove autograft morbidity, they still need sutures at the sites of the repair, which can lead to scar formation and distortion of the repair. Using a conduit at both sites of the repair, we can overcome this limitation and leave both repair sites tension- and suture-free. No studies exist on this technique.
Discussion
Functional outcome is the key determinant of peripheral nerve repairs. This is affected by multiple factors, including mechanism of injury and timing of surgery. Avulsion and crush injuries result in more diffuse axonal injury when compared to lacerations.[32] Repairs are also time-sensitive, with adequate time to allow appropriate healing before motor end-plate degeneration and muscle atrophy.[33] Immediate repair is ideal or within the 3 weeks of injury.[34]
Once a discontinuous nerve injury is identified, surgery involves debridement of non-viable nerve tissue. Proximal and distal neurolysis of the nerve ends can help reduce tension across the repair. This tension-free repair is vital for optimal nerve recovery. Tension across the repair can lead to a severe decrease in blood flow and subsequent ischaemic damage and scar tissue. In animal models, a 15% increase in tension has demonstrated an 80% reduction in blood flow across the anastomotic site.[8] To overcome this, earlier surgical techniques used were techniques such as bone shortening or flexion of a joint to reduce tension, to the detriment of long-term functional rehab due to limb length discrepancies and contractures, respectively.
While autograft has been cited as the gold standard for bridging a nerve gap, it does come with its own sequelae, with both donor site morbidity in the form of paraesthesia or neuroma, essentially moving the deficit from one site to another. Autografts are also limited by availability, calibre, fascicle density and length.[35]
To decrease autograft morbidity, there are now several alternative options including nerve conduits and grafts. However, conduits have demonstrated nerve regenerations in defects up to 30 mm in both animal and human models.[23],[36] Weber et al. showed in a multi-centred randomised control trial that nerve conduits may be better than autograft in peripheral nerve defects. Bertleff et al. prospectively randomised 34 digital nerve lacerations into direct repair to de-tensioned repair with a conduit. No significant difference was seen in the two groups on two-point discrimination.[36]
Conduits can be used in both primary repairs as a tool for de-tensioning and avoiding sutures at the anastomosis and over small nerve gaps.
However, conduits perform less favourably as the defect size of the injury increases, and in these scenarios, allograft is a replacement as a reconstructive option.[37] Allograft allows restoration of nerve continuity, a scaffold for nerve regeneration and no donor morbidity. Allografts and conduits can be used in conjunction in larger gaps using allograft interposition plus connectors and remote sutures. This allows bridging of larger gaps but removes the scaring and bunching caused by sutures at the anastomosis sites
Conclusion
Both allografts and conduits are becoming key tools in the reconstruction of nerve defects and gaps. The off-the-shelf availability of each can facilitate surgeons to confidently debride nerve injuries without fear of the possible nerve gap and reduce over-tensioned repair. Direct repair of a nerve remains the gold standard, but only if it is tension-free throughout the full range of movement of the extremity. As evidence increases on the effectiveness of both of these tools, they will gradually become more used worldwide to reduce the morbidity of autograft. They can also become part of a peripheral nerve algorithm. The various techniques of nerve repair are shown in [Figure - 1] to provide a visual representation of what has been discussed in this review.
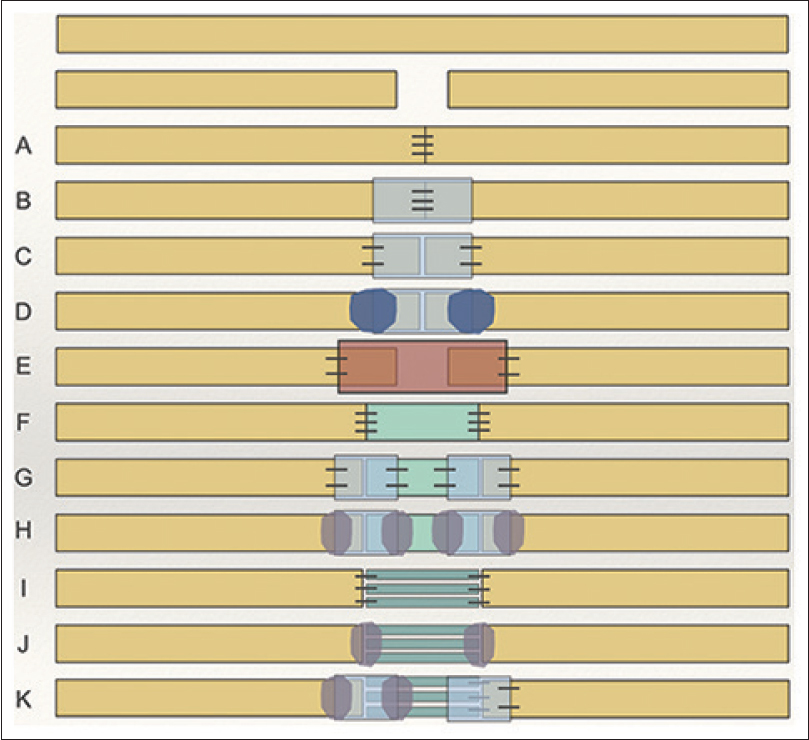 |
| Figure 1: Acute transection injury: A) microsurgical suture; B) suture repair plus connector protection; C) connector assisted “sutureless” neurorraphy; D) connector assisted repair using a polymer glue; E) conduit de-tensioning “gap” repair; F) de-tensioning allograft interposition suture repair; G) de-tensioning allograft interposition connector-assisted “sutureless” repair; H) de-tensioning allograft interposition connector-assisted sutureless repair with fibrin glue; I) de-tensioning autologous nerve graft interposition suture repair; J) de-tensioning autologous nerve graft interposition sutureless repair with fibrin glue; K) Hybrid de-tensioning autologous nerve graft interposition connector assisted repair |
Ethical considerations
Ethical considerations were in keeping with the local hospital ethics board.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Author's contribution
MN, AKN and DP are contributed to the literature search, the structuring of the article and overall writing of the piece including proof reading and editing involved all authors. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Robinson LR. Traumatic injury to peripheral nerves. Muscle Nerve 2000;23:863-73. [Google Scholar] |
| 2. | Wood MD, Kemp SW, Weber C, Borschel GH, Gordon T. Outcome measures of peripheral nerve regeneration. Ann Anat 2011;193:321-33. [Google Scholar] |
| 3. | Heidemann SR, Buxbaum RE. Tension as a regulator and integrator of axonal growth. Cell Motil Cytoskeleton 1990;17:6-10. [Google Scholar] |
| 4. | Ingber DE, Wang N, Stamenovic D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep Prog Phys 2014;77:046603. [Google Scholar] |
| 5. | Dahlin LB. Techniques of peripheral nerve repair. Scand J Surg 2008;97:310-6. [Google Scholar] |
| 6. | Prasad AR, Steck JK, Dellon AL. Zone of traction injury of the common peroneal nerve. Ann Plast Surg 2007;59:302-6. [Google Scholar] |
| 7. | Yi C, Dahlin LB. Impaired nerve regeneration and Schwann cell activation after repair with tension. Neuroreport 2010;21:958-62. [Google Scholar] |
| 8. | Clark WL, Trumble TE, Swiontkowski MF, Tencer AF. Nerve tension and blood flow in a rat model of immediate and delayed repairs. J Hand Surg Am 1992;17:677-87. [Google Scholar] |
| 9. | Zachary LS, Dellon AL. Progression of the zone of injury in experimental nerve injuries. Microsurgery 1987;8:182-5. [Google Scholar] |
| 10. | Williams LR, Longo FM, Powell HC, Lundborg G, Varon S. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: Parameters for a bioassay. J Comp Neurol 1983;218:460-70. [Google Scholar] |
| 11. | Rydevik BL, Kwan MK, Myers RR, Brown RA, Triggs KJ, Woo SL, et al. An in vitro mechanical and histological study of acute stretching on rabbit tibial nerve. J Orthop Res 1990;8:694-701. [Google Scholar] |
| 12. | Danielsen N, Varon S. Characterization of neurotrophic activity in the silicone-chamber model for nerve regeneration. J Reconstr Microsurg 1995;11:231-5. [Google Scholar] |
| 13. | Longo FM, Manthorpe M, Skaper SD, Lundborg G, Varon S. Neuronotrophic activities accumulate in vivo within silicone nerve regeneration chambers. Brain Res 1983;261:109-16. [Google Scholar] |
| 14. | Evans PJ, Bain JR, Mackinnon SE, Makino AP, Hunter DA. Selective reinnervation: A comparison of recovery following microsuture and conduit nerve repair. Brain Res 1991;559:315-21. [Google Scholar] |
| 15. | Merle M, Dellon AL, Campbell JN, Chang PS. Complications from silicon-polymer intubulation of nerves. Microsurgery 1989;10:130-3. [Google Scholar] |
| 16. | Lundborg G, Rosén B, Dahlin L, Holmberg J, Rosén I. Tubular repair of the median or ulnar nerve in the human forearm: A 5-year follow-up. J Hand Surg Br 2004;29:100-7. [Google Scholar] |
| 17. | Inada Y, Hosoi H, Yamashita A, Morimoto S, Tatsumi H, Notazawa S, et al. Regeneration of peripheral motor nerve gaps with a polyglycolic acid-collagen tube: Technical case report. Neurosurgery 2007;61:E1105-7. [Google Scholar] |
| 18. | Archibald SJ, Shefner J, Krarup C, Madison RD. Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J Neurosci 1995;15:4109-23. [Google Scholar] |
| 19. | Liu H, Wen W, Hu M, Bi W, Chen L, Liu S, et al. Chitosan conduits combined with nerve growth factor microspheres repair facial nerve defects. Neural Regen Res 2013;8:3139-47. [Google Scholar] |
| 20. | Meyer C, Stenberg L, Gonzalez-Perez F, Wrobel S, Ronchi G, Udina E, et al. Chitosan-film enhanced chitosan nerve guides for long-distance regeneration of peripheral nerves. Biomaterials 2016;76:33-51. [Google Scholar] |
| 21. | Taras JS, Jacoby SM, Lincoski CJ. Reconstruction of digital nerves with collagen conduits. J Hand Surg Am 2011;36:1441-6. [Google Scholar] |
| 22. | Rinker B, Liau JY. A prospective randomized study comparing woven polyglycolic acid and autogenous vein conduits for reconstruction of digital nerve gaps. J Hand Surg Am 2011;36:775-81. [Google Scholar] |
| 23. | Weber RA, Breidenbach WC, Brown RE, Jabaley ME, Mass DP. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg 2000;106:1036-45. [Google Scholar] |
| 24. | Lundborg G, Dahlin LB, Danielsen N, Gelberman RH, Longo FM, Powell HC, et al. Nerve regeneration in silico ne chambers: Influence of gap length and of distal stump components. Exp Neurol 1982;76:361-75. [Google Scholar] |
| 25. | Porayko MK, Textor SC, Krom RA, Hay JE, Gores GJ, Richards TM, et al. Nephrotoxic effects of primary immunosuppression with FK-506 and cyclosporine regimens after liver transplantation. Mayo Clin Proc 1994;69:105-11. [Google Scholar] |
| 26. | Neubauer D, Graham JB, Muir D. Chondroitinase treatment increases the effective length of acellular nerve grafts. Exp Neurol 2007;207:163-70. [Google Scholar] |
| 27. | Krekoski CA, Neubauer D, Zuo J, Muir D. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J Neurosci 2001;21:6206-13. [Google Scholar] |
| 28. | Johnson PJ, Newton P, Hunter DA, Mackinnon SE. Nerve endoneurial microstructure facilitates uniform distribution of regenerative fibers: A post hoc comparison of midgraft nerve fiber densities. J Reconstr Microsurg 2011;27:83-90. [Google Scholar] |
| 29. | Karabekmez FE, Duymaz A, Moran SL. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand (N Y) 2009;4:245-9. [Google Scholar] |
| 30. | Cho MS, Rinker BD, Weber RV, Chao JD, Ingari JV, Brooks D, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am 2012;37:2340-9. [Google Scholar] |
| 31. | Tang P, Kilic A, Konopka G, Regalbuto R, Akelina Y, Gardner T, et al. Histologic and functional outcomes of nerve defects treated with acellular allograft versus cabled autograft in a rat model. Microsurgery 2013;33:460-7. [Google Scholar] |
| 32. | Isaacs J. Treatment of acute peripheral nerve injuries: Current concepts. J Hand Surg Am 2010;35:491-7. [Google Scholar] |
| 33. | Nath RK, Mackinnon SE. Nerve transfers in the upper extremity. Hand Clin 2000;16:131-9, ix. [Google Scholar] |
| 34. | Kobayashi J, Mackinnon SE, Watanabe O, Ball DJ, Gu XM, Hunter DA, et al. The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve 1997;20:858-66. [Google Scholar] |
| 35. | Millesi H. Techniques for nerve grafting. Hand Clin 2000;16:73-91, viii. [Google Scholar] |
| 36. | Bertleff MJ, Meek MF, Nicolai JP. A prospective clinical evaluation of biodegradable neurolac nerve guides for sensory nerve repair in the hand. J Hand Surg Am 2005;30:513-8. [Google Scholar] |
| 37. | Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DA, Magill CK, et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve 2009;39:787-99. [Google Scholar] |
Fulltext Views
5,540
PDF downloads
2,502





