Translate this page into:
Nerve allograft reconstruction of digital neuromata
Corresponding Author:
Kathryn E Dickson
40 Botyl Road, Botolph Claydon, Bucks, MK18 2LR
UK
katie.e.dickson@gmail.com
| How to cite this article: Dickson KE, Jordaan PW, Mohamed D, Power DM. Nerve allograft reconstruction of digital neuromata. J Musculoskelet Surg Res 2019;3:116-122 |
Abstract
Objective: A symptomatic digital neuroma may have a devastating impact on a person's life. There is no gold standard method of treatment. We hypothesise that the reconstruction of neuroma with nerve allograft will reduce the sensitisation and cortical reorganisation associated with peripheral nerve injury. In this study, we aim to assess the effect of neuroma reconstruction with nerve allograft on patient-reported pain and satisfaction. Methods: We conducted a retrospective review of patients who underwent nerve allograft reconstruction for painful digital neuroma at our unit, from July 2015 to July 2018. We measured pre- and post-operative visual analogue scale (VAS) pain scores, patient satisfaction and patient evaluation measure (PEM) scores. Results: In 10 patients, we reconstructed 12 neuromata. In nine of these patients (11 neuromata), we demonstrated a post-operative reduction in pain, with a change in median VAS score from 7.5 to 1. Patients were satisfied with their operation, with a median satisfaction score of 10/10. The procedure was unsuccessful in two patients, one with a static VAS score and one with a satisfaction score of <8/10, giving a success rate of 80%. Conclusion: Our results show that neuroma reconstruction with nerve allograft can improve patient-reported pain with a success rate of 80%. In our unit, this has become a primary indication for the use of allograft.
Introduction
A neuroma is a result of a nerve's inflammatory response to injury. The majority of neuromata are asymptomatic, but in approximately 3%–5% of cases, it will become painful.[1] A painful neuroma can have a devastating impact on a person's daily function and emotional well-being.[2] There is currently no gold standard of treatment, with management dependent on the nature and anatomy of the neuroma.[3],[4],[5]
Structurally, a neuroma is a large number of small diameter unmyelinated nerve fibres and a chaotic overgrowth of perineural cells.[6] Neuromata form at the end of a cut nerve as an 'end neuroma', or after partial injury within the nerve itself as a 'neuroma in-continuity'. Clinically, a painful neuroma will present with neuropathic pain. The pain can typically be located to a discrete area, which may elicit a positive Tinel's sign, and is relieved temporarily by a local anaesthetic block. The mechanisms through which neuromata become painful are not fully understood but are believed to be a combination of persistent and abnormal peripheral stimulation and altered central processing of pain.[4],[5]
Neuroma management is complex, with >150 treatment options described in the literature.[7] Management is divided broadly into non-surgical and surgical. Non-surgical options consist of both oral and topical analgesics, which can provide acceptable pain relief in their entirety, provide bridging therapy until surgery and in some cases become a useful adjunct to surgery.
In a majority of cases, surgery is the mainstay of treatment and aims to prevent further neuroma formation or to alter the mechanical or chemical environment of the neuroma. Treatment options include neuroma resection[8] with or without reconstruction,[9],[10] neuroma resection and relocation,[9],[11],[12],[13],[14],[15] nerve wrapping or containment, and for neuroma in-continuity, 'neurolysis',[5] with or without wrapping.[5],[16],[17]
The current surgical management strategies for neuroma address the physical environment of the nerve and persistent abnormal stimulation. However, neuroma pain is not just a by-product of the overgrown, disorganised nerve endings but is also a nerve lesion with associated central and peripheral sensitisation[18] and reorganisation of the somatosensory cortex.[19],[20],[21] We hypothesise that nerve reconstruction after neuroma transection will reduce cortical reorganisation and associated pain. A similar principle is used in targeted muscle and sensory re-innervation (TMR), which provides a target for transected nerves in an amputee. TMR has been proven to alter cortical reorganisation[22] and reduce neuroma pain in the upper limb amputees.[23] We believe nerve reconstruction will have a similar effect.
In a patient who already has evidence of peripheral and central sensitisation, the harvesting of nerve autograft has the potential for further neuropathic pain and neuroma formation. To prevent further injury at another site, processed nerve allograft is a possible solution.
Processed nerve allograft (AVANCE® Nerve Graft; AxoGen Inc., Alachua, FL, USA) is decellularised human nerve tissue that maintains the microarchitecture of in vivo nerve tissue with a high density of endoneurial tubes and a basement membrane depleted of neurotoxic glycoproteins. When grafted, the allograft is revascularised and repopulated with host cells, creating an environment conducive to axonal regeneration. Use of allograft in repair of a human peripheral nerve gap has been shown to be safe and effective in restoring sensibility.[24],[25] Use of allograft reconstruction to relieve neuroma pain has not yet been examined.
In our unit, nerve allograft reconstruction is considered in patients with a painful digital neuroma who are deemed to have considerable pain sensitisation. The primary aim of this surgery is pain reduction and not sensory re-innervation. We undertook this study primarily to assess the efficacy of this procedure to improve pain and secondarily to assess acceptability and patient satisfaction with nerve allograft as a treatment modality. This is the largest reported case series of digital neuromas that have been managed with nerve allograft reconstruction, with previous studies reporting on the lower limb.
Materials and Methods
To identify the study cohort, we undertook a retrospective review of patients at our unit who underwent allograft reconstruction for the purpose of neuroma pain relief. AVANCE® Nerve Allograft (AxoGen Inc.) has been used at our unit since July 2015, and in accordance with the Human Tissue Act 2004, patient details are recorded within a trust database. Patients were recruited to the study through interrogation of this database. Inclusion criteria were use of nerve allograft to treat a painful common digital or true digital neuroma; patient to be older than 18 years; a minimum follow-up of 6 weeks; contactable by telephone; and willingness to consent to be involved in the study. Exclusion criteria were any patient with insufficient follow-up or uncontactable by telephone.
We collected demographic data, injury history, operative findings, visual analogue scale (VAS) pain scores, patient satisfaction scores (0–10) and post-operative patient evaluation measure (PEM) scores.[26] These were collected through our unit's electronic records system and a telephone interview.
Surgical technique
The most important aspect of this procedure was pre-operative evaluation with an in-depth discussion with the patient regarding the treatment. In particular, the use of allograft versus autograft was discussed in detail. Since the commencement of this study, the National Institute for Health and Care Excellence (NICE) in the UK has reviewed the use of processed nerve allograft for nerve repair and has provided guidance (IPG: 597), with a minimum audit dataset and clinical governance criteria regarding provision of patient efficacy data before use of nerve allograft, and our unit is compliant with these guidelines.[27]
The indication for surgery in these patients was a painful neuroma, and therefore, the primary aim of surgery was pain relief and not sensory recovery. Allograft reconstruction was only considered in patients with pain at the site of a repaired digital nerve with no recovery or a poor sensory recovery indicating a neuroma in continuity. In patients who had some useful protective sensation, neuroma excision and nerve reconstruction were only performed when the pain at the repair site was severe. These patients consented for a neurolysis and collagen nerve wrap if significant extraneural scar tether and minimal neuroma were identified with intraoperative option to proceed to a neuroma excision if the neuroma was extensive. They were informed that if the neuroma was excised and reconstructed with nerve allograft, the sensory recovery may be no better or worse than the sensation before surgery and that the main intended benefit from surgery was the alleviation of nerve pain. In these cases, the use of a local anaesthetic block performed preoperatively proximally on the affected nerve could confirm relief of pain and simulate an insensate finger should surgery not result in sensory recovery in a patient with some useful sensation before surgery.
Surgery was performed under axillary nerve block or general anaesthetic. Using loupe magnification, the neuroma was identified and neurolysis of the affected nerve [Figure - 1] was carried out. An operating microscope was used to excise the neuroma and to debride the proximal and distal nerve ends with a neurotome. Satisfactory debridement was achieved by confirming a healthy fascicular pattern with minimal interfascicular scar and vascularised nerve ends with bleeding at the site of neurotomy [Figure - 2].
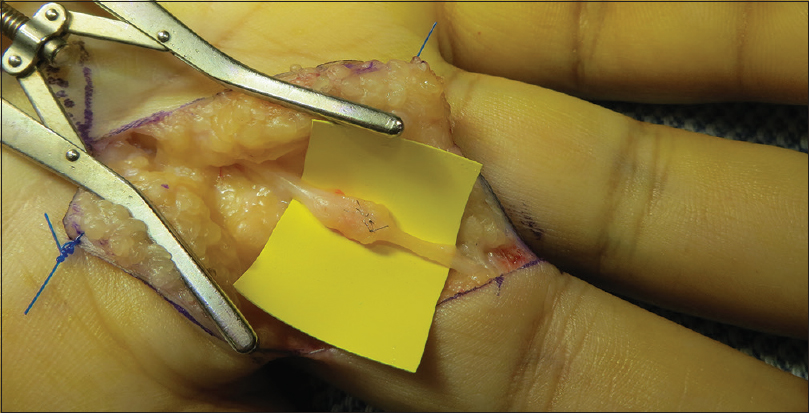 |
| Figure 1: A radial digital nerve neuroma in-continuity to the left middle finger, sustained following delayed nerve repair for trauma at a referring hospital. The nerve was tethered in the scar of the previous repair |
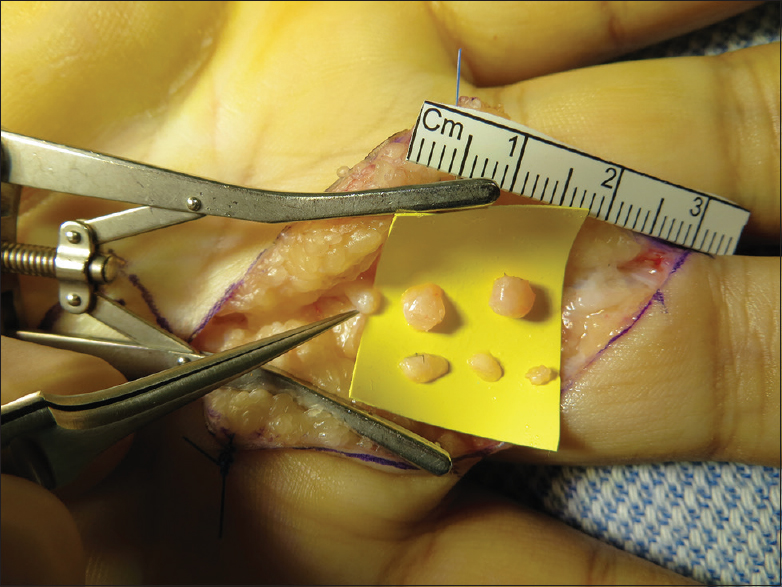 |
| Figure 2: The neuroma was excised with a neurotome in sequential slices to healthy fascicles, with a resultant defect of 18 mm |
The nerve gap was measured with the fingers in full extension and the appropriately sized AVANCE® processed nerve allograft was prepared as per the manufacturer's instructions. Allograft is supplied frozen and was thawed in warm saline, trimmed to the correct length to interpose in the nerve gap in full extension of the digit and sutured in place. Tension-free neurorrhaphy was performed with three interrupted 9-0 non-absorbable epineural sutures under microscope magnification at each neurorrhaphy site [Figure - 3].
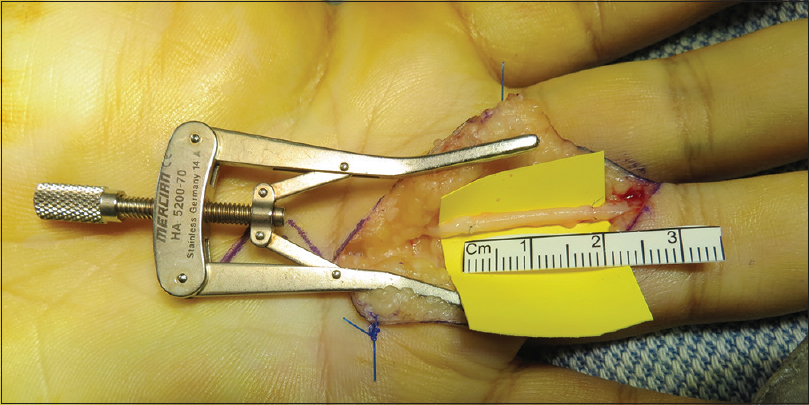 |
| Figure 3: The nerve gap was measured with the fingers in full extension, and using a 20-mm length of 2–3 mm diameter AVANCE nerve allograft, trimmed to length, and tension-free nerve repair was performed |
The hand was splinted in a functional position for the first few days after which early active range of motion was encouraged. Patients attended a specialist hand surgery dressing clinic within seven to 14 days for a wound check and hand therapist review. Follow-up was in a specialist peripheral nerve clinic at 6 weeks, 3 months and 6 months following surgery.
Results
Eleven patients underwent allograft reconstruction for digital nerve neuromata, of which 10 patients had sufficient follow-up data at the time of writing. Six cases followed trauma surgery (volar approach for a metacarpal-phalangeal joint dislocation, amputation following high-pressure injection, flap reconstruction of a thumb and digital nerve repair in three cases), three cases were a delayed presentation following trauma and one case was a complication of elective surgery (carpal tunnel decompression) [Table - 1] and [Table - 2].

In 10 patients, 12 neuromas were reconstructed. The indication for surgery in all of the cases was a sensitised, painful neuroma of the digital nerve or common digital nerve [Table - 3].
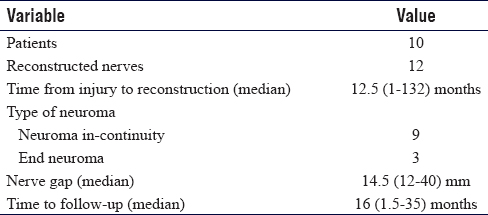
Pain was measured using a 10-point VAS with 0 indicating no pain and 10 the worst pain imaginable [Table - 4] and [Table - 5]. One patient (Patient 7) did not have a preoperative VAS score. This patient's post-operative pain score was 4/10 with a satisfaction of 10/10.
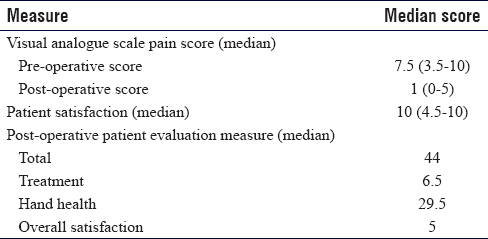
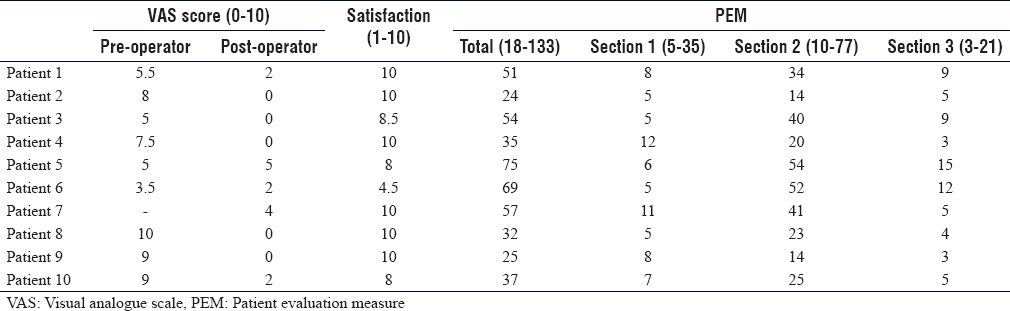
The median preoperative pain score was 7.5 (range 3.5–10; n = 9), improving to 1 (range 0–5; n = 8) post-operatively. Two patients were still using analgesia at last follow-up. Five patients scored their pain as 0/10 post-operatively.
In one patient (Patient 5), there was no improvement in VAS score [Table - 5]. This patient had to change to lighter duties at work and was still using regular analgesia at last follow-up.
Post-operative satisfaction was measured on a 10-point scale where patients rated their general satisfaction, with 0 indicating complete dissatisfaction and 10 complete satisfaction. Median patient satisfaction was 10 (range 4.5–10).
A post-operative PEM questionnaire was undertaken with each patient during a telephone interview. The PEM score was designed as a measure of overall hand health, with three sections looking at a patient's general experience of their treatment (range 5–35), hand health (range 10–77) and overall satisfaction (range 3–21). Questions are answered on a scale of 1–7, with 1 being the positive score and 7 the most negative. Sections are analysed together to give a total score (range 18–135). A lower score indicates greater patient satisfaction. The median total postoperative PEM was 44, with the median treatment score 6.5, median hand health score 29.5 and median overall satisfaction 5.
Eight patients were working pre-operatively and all eight returned to work, but two patients returned to lighter duties. The latest return to work was 2 months following surgery in a manual labourer who returned to his regular work.
To date, we have not seen any infection or evidence of failure of the allograft to support neural regeneration. No patient has undergone revision surgery. There has not been a recurrence of neuroma following allograft reconstruction. The patient with no VAS improvement (Patient 5) has not been confirmed as a recurrence of neuroma.
Two cases were non-anatomical reconstructions. Patient 9 developed a stump neuroma following a ray amputation and had a loop anastomosis performed from the radial digital to ulnar digital nerve using a section of allograft. Patient 7 developed a neuroma following a flap reconstruction of the thumb and had allograft grafted to the excised neuroma proximal stump and the distal end of the allograft routed proximally as a “graft to nowhere” with no distal neurorrhaphy.
Discussion
The aim of our series was to assess whether nerve allograft is effective in reducing pain in digital neuromata. With a median follow-up of 16 months, in 10 patients, all but one reported improved VAS score post-operatively, with a decrease in median score from 7.5 to 1.
The operation failed to alleviate pain in one patient, Patient 5, whose pre- and post-operative VAS score remained at 5. Without further exploratory surgery, it is not possible to explain this failure. Hypothetically, the repair may have failed and neuroma recurred, the neuroma may not have been fully debrided, or neuroma may not have been the initial cause of pain.
One patient, Patient 6, had a relatively small improvement in VAS score, and the lowest satisfaction score of 4.5. This patient was a victim of assault, sustaining a right index finger radial digital nerve injury. The patient initially refused repair, but after developing a painful sensitised neuroma underwent neuroma excision and reconstruction. This patient had the lowest initial preoperative VAS score and underwent neuroma reconstruction only a month after initial injury, the shortest time period from injury to surgery in the group. It is possible that this was not long enough for cortical reorganisation to have taken effect. In the pain literature, central sensitisation is thought to occur after months of living with pain.[28],[29] This may explain this patient's initial low VAS score and lack of improvement. Of note, Patient 6 returned to regular work, and had an overall PEM score of 69.
Our secondary outcomes are also positive. The majority of patients described themselves as satisfied with the operation, with a median score of 10/10. Only one patient had a satisfaction score of <8; Patient 6, who has been discussed above. Median total postoperative PEM score was 44. Sub-analyses of the different sections show good results with a median score of 6.5 for general experience of treatment, 29.5 for hand health and overall satisfaction of 5.
Other authors have used the Disability of the Arm, Shoulder and Hand (DASH) questionnaire to assess patient-reported outcomes, showing that pre- and post-operative DASH scores improve with surgery.[17],[28],[30] The Impact of a Hand Nerve Disorders scale (I-HaND) is a new patient-reported outcome measure for nerve disorders in hand and should be validated for the repair of digital nerves and chronic neuroma pain necessitating nerve reconstruction with autograft and allograft.[31]
Objective measures are also promising, with only two patients remaining on oral analgesics, and all eight working preoperatively able to return to work, albeit with two to lighter duties. To date, we have seen no complications in our patient group and no revision operations.
In the authors' opinion in our series, two cases would be described as a failure of surgical treatment (Patients 5 and 6). Of 12 neuromas in 10 patients, ten neuromas and eight patients have been treated successfully. Our success rate was 80% in this case series. A recent meta-analysis of surgical intervention for the treatment of painful neuroma found that the proportion of patients with a meaningful reduction in neuroma pain following surgery was 77%, regardless of the surgical technique.[32] Our technique performs comparably.
There are limitations to our study. It is small in size, a reflection of the novelty of the procedure and the low prevalence of painful neuroma. This procedure is only offered to patients with a painful neuroma and either insensate skin or diminished protective sensation. This is a small subgroup of our nerve injury caseload.
Pre-operative VAS scores were collected retrospectively, rather than prospectively, and so may be affected by recollection bias. This was as a consequence of study design, but also reflects the difficulty of studying pain. Pain is a subjective, variable experience that is difficult to quantify.[33],[34] We believe that a retrospective assessment of pain allows us to assess the patient's subjective experience of their improvement in their pain.
The post-operative PEM score includes an assessment of a patient's general experience of their management as well as overall satisfaction with their treatment. Even though we cannot measure improvements in hand health, we were able to measure patient experience of treatment and overall satisfaction. We choose the PEM score over DASH as a measure of patient-reported outcomes because in hand it has been shown to have a greater responsiveness to change.[35]
Processed nerve allograft is not available in all centres. It has only recently been approved by the NICE (IPG: 597)[27] with sufficient evidence to support use in digital nerve repair and a requirement for enhanced governance, audit and research for non-digital sensory nerves and for mixed motor-sensory nerves based on the more limited published outcomes in these latter two areas.[27] Currently, it is used at only a few centres in the UK, is costly in comparison to other described methods of neuroma management and remuneration systems are locally negotiated due to a lack of a UK national coding and tariff.
Further research should concentrate on long-term efficacy, reduction in pain, improvements in Elliot neuroma scores,[9] validation of the I-HaND in this patient group[31] use of functional MRI to assess cortical reorganisation and qualitative research.
Allograft reconstruction should be carefully considered in patients with painful digital neuromata and only after conservative measures have been exhausted. Treatment options should be discussed in detail with the patient, and the decision to use allograft should be considered when pain sensitisation is apparent. Local anaesthetic nerve blocks can evaluate the source of pain and surgical targets. According to current NICE guidelines in the UK the patient should be provided with written guidance on processed nerve allograft detailing the evidence base and inviting the patient to be involved in prospective audit data collection. The recommended outcome measures are available on the NICE website.
There are important potential benefits to using processed nerve allograft. It negates the creation of a donor site, reducing the risk of further neuroma development in a potentially already sensitised patient. It also negates the resultant donor site deficit. The use of processed nerve allograft reduces the surgical field, allows for regional anaesthesia and single limb operating, can significantly reduce operative time, avoids donor site complications and provides comparable results in digital nerve reconstruction compared to autograft in a prospective study[24] and the RANGER registry study.[25]
Conclusion
Painful digital neuromata present a complex and challenging surgical problem. They are frequently life-changing, causing disabling pain and impact on psychosocial well-being. We present a novel method for surgical management of neuroma pain that attempts to address the cortical reorganisation seen in peripheral nerve injury while avoiding donor site morbidity in a sensitised patient. It has been successful in reducing pain and providing satisfaction in 80% of our treated patients, without any complications to date. In our unit, this has become a primary indication for the use of processed nerve allograft.
Ethical approval
Ethical approval to contact patients to collect outcome data is covered by their inclusion in the RANGER study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
The HaPPeN research team at UHB is an international recruitment centre for the RANGER study and the senior author is the Chief Investigator for RANGER in the UK. The HaPPeN team receives financial support from AxoGen for collecting outcome data in nerve surgery for the RANGER study.
Author's contribution
DP conceived and designed the study and provided research materials. KED collected and organised data, and wrote initial and final draft. PWJ analysed and interpreted data. DM collected data. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Sunderland S. Nerves and Nerve Injuries. 1st ed. Edinburgh: Livingstone; 1968. [Google Scholar] |
| 2. | Wojtkiewicz DM, Saunders J, Domeshek L, Novak CB, Kaskutas V, Mackinnon SE, et al. Social impact of peripheral nerve injuries. Hand (N Y) 2015;10:161-7. [Google Scholar] |
| 3. | Jordaan P, Wang CK, Ng CY. Management of painful cutaneous neuromas around the wrist. Orthop Trauma 2017;31:290-5. [Google Scholar] |
| 4. | Watson J, Gonzalez M, Romero A, Kerns J. Neuromas of the hand and upper extremity. J Hand Surg Am 2010;35:499-510. [Google Scholar] |
| 5. | Laing T, Siddiqui A, Sood M. The management of neuropathic pain from neuromas in the upper limb: Surgical techniques and future directions. Plast Aesthet Res 2015;2:165. [Google Scholar] |
| 6. | Cravioto H, Battista A. Clinical and ultrastructural study of painful neuroma. Neurosurgery 1981;8:181-90. [Google Scholar] |
| 7. | Wood VE, Mudge MK. Treatment of neuromas about a major amputation stump. J Hand Surg Am 1987;12:302-6. [Google Scholar] |
| 8. | Herrmann LG, Gibbs EW. Phantom limb pain. Am J Surg 1945;67:168-80. [Google Scholar] |
| 9. | Elliot D, Sierakowski A. The surgical management of painful nerves of the upper limb: A unit perspective. J Hand Surg Eur Vol 2011;36:760-70. [Google Scholar] |
| 10. | Sosin M, Weiner LA, Robertson BC, DeJesus RA. Treatment of a recurrent neuroma within nerve allograft with autologous nerve reconstruction. Hand (N Y) 2016;11:NP5-9. [Google Scholar] |
| 11. | Herndon JH, Eaton RG, Littler JW. Management of painful neuromas in the hand. J Bone Joint Surg Am 1976;58:369-73. [Google Scholar] |
| 12. | Mass DP, Ciano MC, Tortosa R, Newmeyer WL, Kilgore ES Jr. Treatment of painful hand neuromas by their transfer into bone. Plast Reconstr Surg 1984;74:182-5. [Google Scholar] |
| 13. | Sood MK, Elliot D. Treatment of painful neuromas of the hand and wrist by relocation into the pronator quadratus muscle. J Hand Surg Br 1998;23:214-9. [Google Scholar] |
| 14. | Hazari A, Elliot D. Treatment of end-neuromas, neuromas-in-continuity and scarred nerves of the digits by proximal relocation. J Hand Surg Br 2004;29:338-50. [Google Scholar] |
| 15. | Goldstein SA, Sturim HS. Intraosseous nerve transposition for treatment of painful neuromas. J Hand Surg Am 1985;10:270-4. [Google Scholar] |
| 16. | Masear VR, Colgin S. The treatment of epineural scarring with allograft vein wrapping. Hand Clin 1996;12:773-9. [Google Scholar] |
| 17. | Thomsen L, Bellemere P, Loubersac T, Gaisne E, Poirier P, Chaise F, et al. Treatment by collagen conduit of painful post-traumatic neuromas of the sensitive digital nerve: A retrospective study of 10 cases. Chir Main 2010;29:255-62. [Google Scholar] |
| 18. | Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983;306:686-8. [Google Scholar] |
| 19. | Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M, et al. Massive cortical reorganization after sensory deafferentation in adult macaques. Science 1991;252:1857-60. [Google Scholar] |
| 20. | Lotze M, Grodd W, Birbaumer N, Erb M, Huse E, Flor H, et al. Does use of a myoelectric prosthesis prevent cortical reorganization and phantom limb pain? Nat Neurosci 1999;2:501-2. [Google Scholar] |
| 21. | Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 1995;375:482-4. [Google Scholar] |
| 22. | Serino A, Akselrod M, Salomon R, Martuzzi R, Blefari ML, Canzoneri E, et al. Upper limb cortical maps in amputees with targeted muscle and sensory reinnervation. Brain 2017;140:2993-3011. [Google Scholar] |
| 23. | Souza JM, Cheesborough JE, Ko JH, Cho MS, Kuiken TA, Dumanian GA, et al. Targeted muscle reinnervation: A novel approach to postamputation neuroma pain. Clin Orthop Relat Res 2014;472:2984-90. [Google Scholar] |
| 24. | Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, et al. Processed nerve allografts for peripheral nerve reconstruction: A multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery 2012;32:1-4. [Google Scholar] |
| 25. | Cho MS, Rinker BD, Weber RV, Chao JD, Ingari JV, Brooks D, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am 2012;37:2340-9. [Google Scholar] |
| 26. | Macey AC, Burke FD, Abbott K, Barton NJ, Bradbury E, Bradley A, et al. Outcomes of hand surgery. J Hand Surg 1995;20:841-55. [Google Scholar] |
| 27. | National Institute for Health and Care Excellence. Processed Nerve Allografts to Repair Peripheral Nerve Discontinuities,Guidance and Guidelines. Available from: https://www.nice.org.uk/guidance/ipg597/chapter/1-Recommendations. [Last accessed on 2018 Oct 04]. [Google Scholar] |
| 28. | Domeshek LF, Krauss EM, Snyder-Warwick AK, Laurido-Soto O, Hasak JM, Skolnick GB, et al. Surgical treatment of neuromas improves patient-reported pain, depression, and quality of life. Plast Reconstr Surg 2017;139:407-18. [Google Scholar] |
| 29. | Cohen SP, Mao J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014;348:f7656. [Google Scholar] |
| 30. | Guse DM, Moran SL. Outcomes of the surgical treatment of peripheral neuromas of the hand and forearm: A 25-year comparative outcome study. Ann Plast Surg 2013;71:654-8. [Google Scholar] |
| 31. | Ashwood M, Jerosch-Herold C, Shepstone L. Development and validation of a new patient-reported outcome measure for peripheral nerve disorders of the hand, the I-haND© scale. J Hand Surg Eur Vol 2018;43:864-74. [Google Scholar] |
| 32. | Poppler LH, Parikh RP, Bichanich MJ, Rebehn K, Bettlach CR, Mackinnon SE, et al. Surgical interventions for the treatment of painful neuroma: A comparative meta-analysis. Pain 2018;159:214-23. [Google Scholar] |
| 33. | Badalamente M, Coffelt L, Elfar J, Gaston G, Hammert W, Huang J, et al. Measurement scales in clinical research of the upper extremity, part 1: General principles, measures of general health, pain, and patient satisfaction. J Hand Surg Am 2013;38:401-6. [Google Scholar] |
| 34. | Cruccu G, Sommer C, Anand P, Attal N, Baron R, Garcia-Larrea L, et al. EFNS guidelines on neuropathic pain assessment: Revised 2009. Eur J Neurol 2010;17:1010-8. [Google Scholar] |
| 35. | Hobby JL, Watts C, Elliot D. Validity and responsiveness of the patient evaluation measure as an outcome measure for carpal tunnel syndrome. J Hand Surg Br 2005;30:350-4. [Google Scholar] |
Fulltext Views
4,404
PDF downloads
753





