Translate this page into:
Patterns of injury to the infraclavicular brachial plexus following dislocation of the glenohumeral joint
2 Clinical Neurophysiology, Queen Elizabeth Hospital Birmingham, Birmingham, United Kingdom
3 Royal Centre of Defence Medicine, University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom
4 Birmingham Hand Centre, University Hospitals Birmingham NHS Foundation Trust, Birmingham, United Kingdom
Corresponding Author:
Isabel A Guy
6 Station Road, Birmingham, West Midlands B17 9JT
United Kingdom
isabelguy@hotmail.co.uk
| How to cite this article: Guy IA, Guerero DN, Shirley CP, Edwards CJ, Miller C, Power DM. Patterns of injury to the infraclavicular brachial plexus following dislocation of the glenohumeral joint. J Musculoskelet Surg Res 2019;3:90-97 |
Abstract
Objectives: Brachial plexus injury (BPI) often results in devastating loss of upper limb function and debilitating neuropathic pain. The medial cord is often damaged following low-energy falls and glenohumeral dislocation. Medial cord injury (MCI) is associated with poor functional hand outcome due to paralysis of intrinsic muscles innervated by median and ulnar nerves, yet management options are limited. Nerve injury severity, demographic factors and concomitant injuries are poorly defined in this group. This study aims to understand patterns of infraclavicular BPI to guide management. Methods: All consecutive cases of infraclavicular BPI presenting to a regional peripheral nerve injury service over a 3-year period were retrospectively analysed. Medical records and neurophysiology reports were reviewed, and demographics and injury details were recorded on a database. Results: Ninety-nine infraclavicular BPI cases were identified. Of these, 34 (34%) were attributed to glenohumeral dislocations sustained in low-energy falls. There were 21 females and 13 males with mean age 62 years and mean body mass index 31. Five (13%) and 11 (29%) patients sustained vascular injuries and rotator cuff tears, respectively. Twelve (35%) patients sustained 13 fractures, of the proximal humerus or greater tuberosity. Review of injury patterns identified MCI in 24 cases (71%), 12 (50%) of which were amenable to nerve transfers. Conclusion: Low-energy falls are often accompanied by glenohumeral dislocation, whereby the medial cord is commonly damaged, resulting in an intrinsic minus hand. This injury subgroup has not been previously described, yet early recognition and referral for novel nerve transfer surgery can improve outcomes for these life-changing injuries.
Introduction
Brachial plexus injury (BPI) often results in devastating disturbance of upper limb function, restriction of activities of daily living performance,[1] severe neuropathic pain and broad socioeconomic ramifications for both the individual and their family.[2]
Infraclavicular BPI is an uncommon injury associated with both low- and high-energy trauma. There are age-related peaks of incidence in young males involved in motorcycle or other road traffic collisions (RTCs) and in elderly patients sustaining low-energy traumatic falls. Glenohumeral dislocation, one of the most common joint dislocations owing to the mobility of the glenohumeral joint, was first recognised as a causative mechanism of infraclavicular BPI by Delbe and Cauchoix in 1910.[3] [Figure - 1] and [Figure - 2] demonstrate the mechanism by which glenohumeral dislocation compromises integrity of the infraclavicular plexus. Closed low-energy infraclavicular BPIs remain a frustrating surgical dilemma. Although some patients will have good spontaneous recovery from these injuries, many will be left with poor functional outcomes and no useful grip or intrinsic muscle function of the hand.[4]
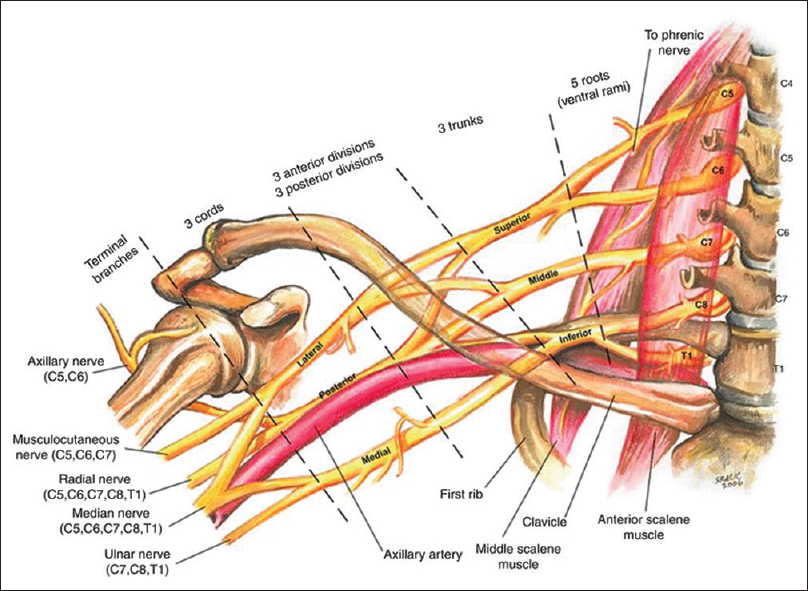 |
| Figure 1: Anatomy of the brachial plexus. Buckenmaier C, Bleckner L, Sracic M. Military-advanced regional anaesthesia and analgesia handbook. 1st ed. Washington: Borden Institute; 2009. p. 22; Figure 5 and 2 |
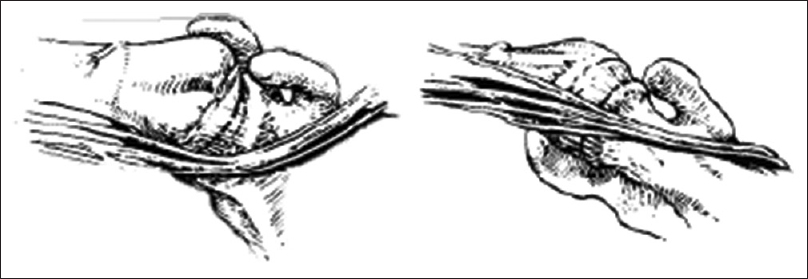 |
| Figure 2: Frontal and caudal views of relationships between the axillary nerve, the infraclavicular neurovascular bundle and the humeral head in internal rotation. Both axillary nerve and neuromuscular bundle are caudal to the humeral head and stretched over it. Coene L. Mechanisms of brachial plexus lesions. Clin Neurol Neurosurg 1993;95:24-9 |
High BPIs, where the medial cord is injured proximally, result in notoriously poor outcomes with regard to recovery of intrinsic hand muscles because of two fundamental principles:
- Axonal regeneration is limited to 1–2 mm/day
- The majority of motor endplates remain responsive to reinnervation for 12 months only.[5]
By the time regenerating axons have covered the long distance from the point of injury to the median and ulnar nerve targets located in the hand and distal forearm, muscles have atrophied and motor endplates are unresponsive. Compared to the four other terminal branches of the plexus, the ulnar nerve has been found to have the lowest rate of good recovery (50%).[6]
Hems' study of outcomes in patients who sustained infraclavicular BPI following glenohumeral dislocation found that full recovery was attained in all damaged axillary and musculocutaneous nerves. However, full recovery was attained in only 17% of median nerves and 11% of ulnar nerves. This study concluded that outcomes for ulnar nerve injuries at the level of the glenohumeral are unlikely to be improved by any form of repair; this emphasises the medial cord problem.[7]
Medial cord injuries (MCIs) involve loss of active digital flexion and fine hand control through the intrinsic muscles, impairing grip and dextrous hand function. Such functional loss has considerable socioeconomic consequences; in a group of 81 ulnar and/or median nerve-injured patients, the mean time off work exceeded 31 weeks; furthermore, within 1 year after combined nerve injuries, only 24% (versus 80% after isolated median and 59% after ulnar nerve injuries) returned to work.[8]
With an increasing elderly population worldwide – the UK population in which those aged 65 years and over are predicted to comprise almost 25% by 2046,[9] the incidences of falls and BPIs is predicted to increase.[10] Infraclavicular BPIs remain a challenging problem with a paucity of published evidence. It is difficult to make direct comparisons between studies due to heterogeneity in terms of patient populations, injury pathomechanics, classification of injury patterns, management and outcomes reporting.[11] A comprehensive analysis of the patterns of infraclavicular BPI is indicated, in order for peripheral nerve surgeons to advance clinical practice and identify early predictors of poor outcome and to explore novel treatment options.
The principal aims of this study are to (1) understand the demographics of patients presenting with infraclavicular injuries, (2) recognise patterns of infraclavicular injuries and (3) identify factors associated with poor prognosis.
Subjects and Methods
Data collection
The study was registered at our institution, and following board review, the researchers were given approval to undertake the data collection and analysis. Two co-researchers reviewed all cases presenting to a regional peripheral nerve injury service from 2012 to 2016 with an assigned diagnostic code 'infraclavicular BPI' by their attending clinician. A total of 100 injuries were identified in 99 patients (a bilateral BPI was sustained by one patient). The medical records, therapy records, imaging and neurophysiology reports were examined, and key variables recorded in a bespoke database were created for this study. These variables included patient demographics, injury features, interventions and outcome of the injury [Table - 1].

Nerve damage
Nerve injury to each of six branches of the brachial plexus (ulnar, musculocutaneous, radial and axillary nerves, and the lateral and medial roots of the median nerve) was graded based on clinical examination findings and electrodiagnostic studies. Grading was based on classification systems established by Seddon, Sunderland and Mackinnon.[5] Injuries were defined as 'no injury', 'neurapraxia', 'axonotmesis', 'neurotmesis' or 'mixed'. An overall consensus on nerve injury grade to each of these branches was achieved by the two researchers, with senior author review where no consensus was reached.
Data interpretation and analysis
Demographic details
Mean, median and range values were calculated for age and body mass index (BMI) using Microsoft Excel. With regard to relevant medical history, the number of patients with a positive history of each factor (diabetes mellitus, smoking and hypertension) was converted into a percentage of all patients in the database.
Injury features
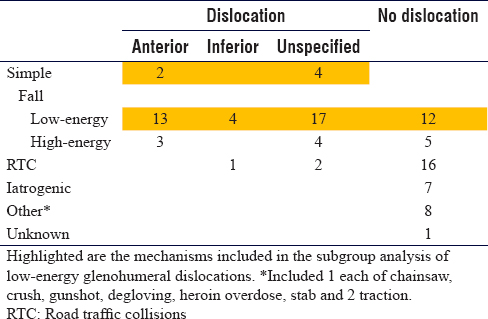
All datasets were organised into categories of causative mechanism, each of which was subdivided into whether shoulder dislocation was present or not [Table - 2]. If shoulder dislocation was present, it was further subdivided into 'anterior', 'inferior' or 'unclassified'. Those patients who were classified as having sustained a dislocation in 'simple' and 'low-energy fall' groups were identified as the population relevant to this study's research question, and data analysis was only conducted for this group.

Regarding time to shoulder reduction, the number and percentage of patients who fell into each group ('early', 'prolonged' and 'late') was calculated. The number of patients with a positive finding of vascular injury, fracture or rotator cuff tear was converted into a percentage. The patterns of nerve damage of all 99 patients for whom sufficient data on degree of nerve injury was present (n = 82) were categorised.
Medial cord outcome
Outcomes for flexor carpi ulnaris, flexor digitorum profundus and intrinsics according to the Medical Research Council (MRC) scale were grouped into 3 categories [Table - 3].

Injury severity scores
Each grade of nerve injury was assigned a severity score [Table - 4]. Patients' overall BPI severity scores were determined by the sum of each score achieved in their respective brachial plexus territories. Demographic and injury details were correlated with these to determine poor prognostic indicators.

Results
Database interrogation identified 100 infraclavicular BPIs during a 3-year period with at least 12-month follow-up. Two of these injuries were sustained by the same patient (bilateral BPI); so, data from 99 patients were retrieved.
Patterns of nerve damage
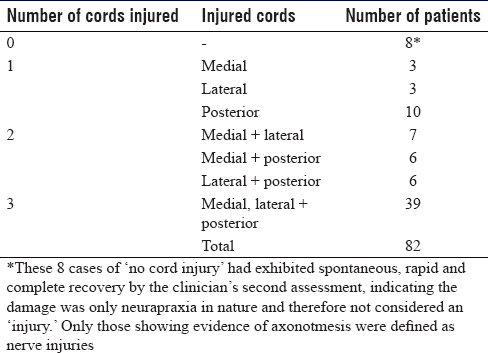
Patterns of nerve damage of all 99 patients in the database for whom sufficient data on the degree of nerve injury was present (n = 82) were categorised [Table - 5].
Causative mechanism
Following exclusion of high-energy injuries, stabs, gunshots and iatrogenic injuries, there were 52 injuries classified as low-energy and a sub-group of 40 cases of confirmed glenohumeral dislocation [Table - 6]. Nerve injury classification data were complete in 34 patients (89%), and these were included in the final analysis [Figure - 3].
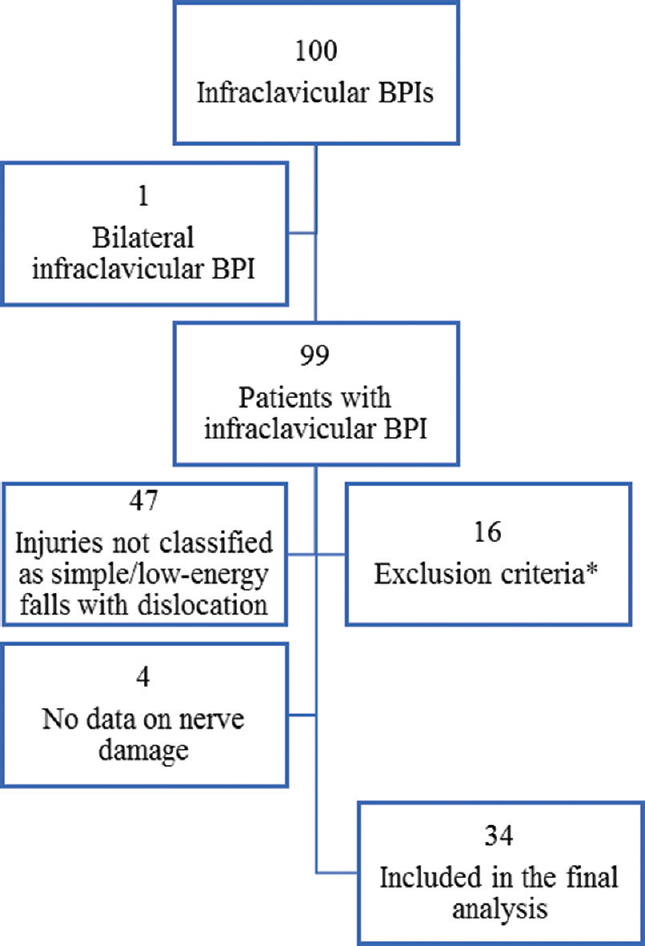 |
| Figure 3: Inclusion and exclusion of cases for the clinical series. *Table 7 for exclusions |
Exclusion
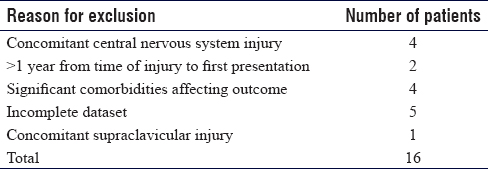
Sixteen datasets were excluded for the reasons listed in [Table - 7], which were considered potential confounders by the researchers. This yielded 83 datasets that qualified for interpretation and analysis.
Demographic details
There were 21 females and 13 males with a mean age of 62 years (range 30–87) and median 63 years. Mean BMI was 31 (20.8–45.5); median BMI was 29. A positive history of diabetes mellitus, smoking and hypertension was found in 27.6%, 41.1% and 44.8% of patients, respectively.
Injury features
Five (13%) patients had a vascular injury and 11 (29%) had rotator cuff tears. Twelve (35%) patients sustained 13 fractures, of which all were of the proximal humerus or greater tuberosity. Time to glenohumeral reduction was reported as early (<2 h) in 12 patients, prolonged (2–24 h) in 9 and late (>24 h) in 9. This information was not recorded for four patients.
Nerve damage
MCI, including those defined as either axonotmesis or mixed, was identified in 24 cases (71%), and half of these patients (n = 12) had injury to all six nerves (ulnar nerve, medial root of median nerve, lateral root of median nerve, musculocutaneous nerve, axillary nerve and radial nerve) and therefore would not qualify as intraplexus nerve transfer candidates due to lack of an intact donor nerve. The 12 injuries with sparing of at least one cord are potentially amenable to nerve transfer surgery for restoration of important motor function to the medial cord [Figure - 4].
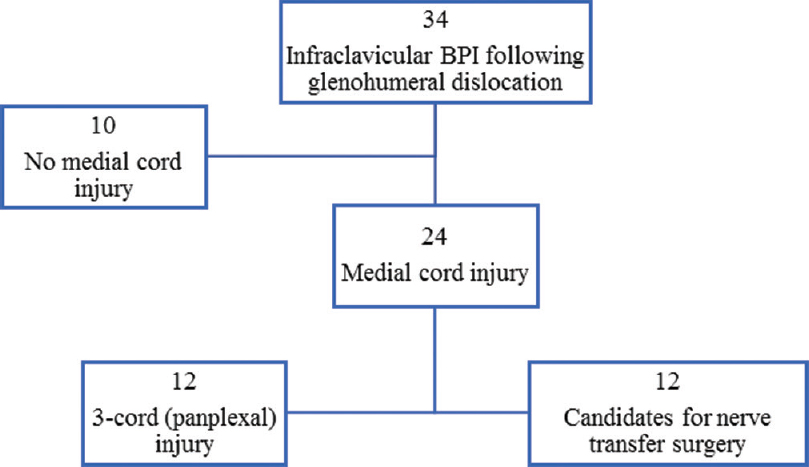 |
| Figure 4: Injury patterns and subsequent amenability to nerve transfer surgery in 34 patients with infraclavicular brachial plexus injury following glenohumeral dislocation |
Injury severity scores
Injury severity score (ISS) was calculated for the 34 patients with infraclavicular BPI following glenohumeral dislocation.
Demographics
Demographic factors were found to be associated with a higher ISS including age over 80 years, female and BMI exceeding 25 [Table - 8] although these were not statistically significant. A positive history of either smoking or hypertension also predicts more severe injury; however, a positive history of diabetes mellitus predicts a lower severity.
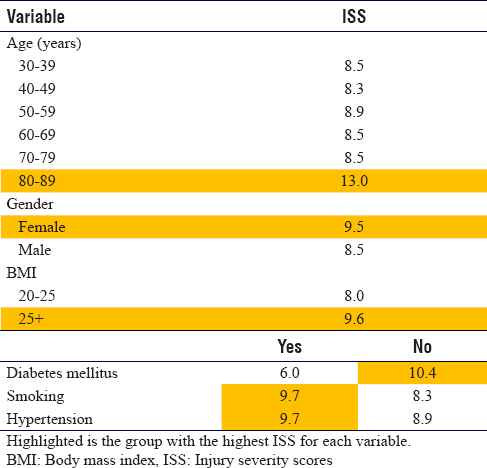
Injury features
Vascular injury, fractures and an absence of rotator cuff tear were found to be associated with more severe injury [Table - 9].

Outcomes for intrinsic hand function
A total of 24 patients with medial cord axonotmesis were identified. In 2 cases, medial cord injuries were limited to the medial root of the median nerve and therefore had sparing of the ulnar nerve; these cases were discounted for this particular analysis. The remaining subgroup of 22 cases is clinically important because the ulnar nerve provides the main contribution to intrinsic hand function. The intrinsic hand motor function outcomes for this group, once any interventions had taken place and at least 12 months after injury, were reported [Figure - 5].
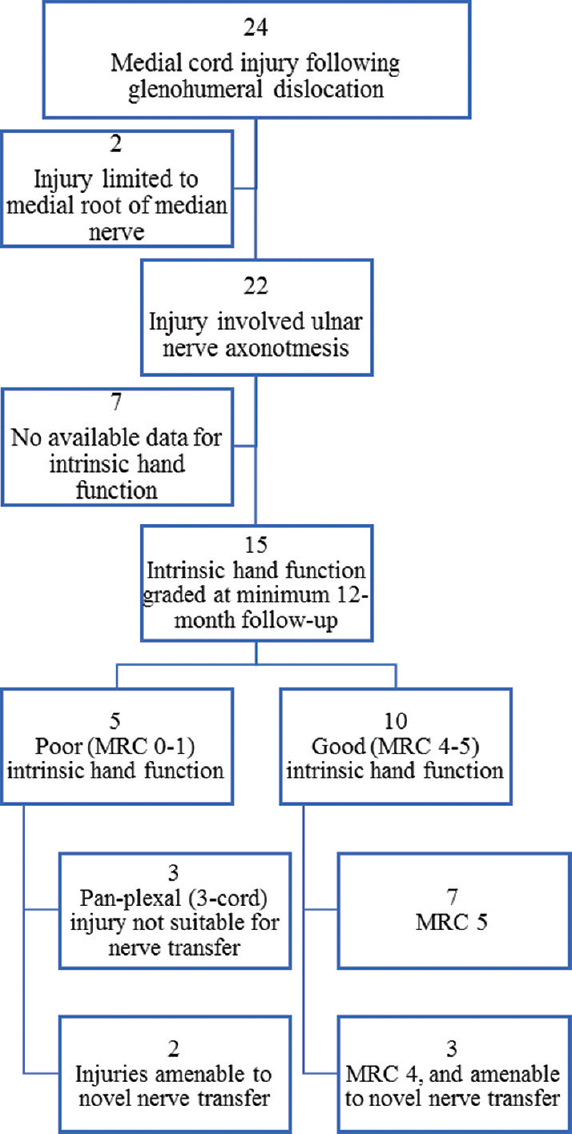 |
| Figure 5: Injury patterns and subsequent amenability to nerve transfer surgery in 24 patients with medial cord injury following glenohumeral dislocation |
Some 10 cases were found to have 'good' intrinsic hand function, with MRC graded 4 or 5, while 5 cases were left with 'poor' function, all of which graded MRC 0. No information on intrinsic hand function was available for 7 cases.
Therefore, 1 in 3 cases who sustained ulnar nerve axonotmesis following glenohumeral dislocation regained no function in their intrinsic hand muscles; instead, they were left with a permanently 'intrinsic-minus hand' characterised by a claw deformity. These patients have weakened grip and grasp strength, resulting in considerably reduced functional hand efficiency that is disabling and life-changing.
Of the 5 intrinsic-minus cases, 3 had sustained pan-plexal injuries so had no available donor nerves, rendering them ineligible for transfer surgery. However, 2 cases might have been amenable for nerve transfers that could have the potential to provide intrinsic reinnervation and improved hand outcome. Closer review of the 10 cases who regained good intrinsics showed that 3 had function graded MRC 4. Therefore, there may have been room for improvement in this group that could be achieved through nerve transfers, for which all these patients' injuries were suitable.
In this way, 8 patients who sustained ulnar nerve axonotmesis did not regain full intrinsic hand function (53%), but it was considered that 5 of these (62.5%) could have had nerve transfer surgery that might have improved their final outcomes.
Prognostic indicators of intrinsic recovery
Fifteen cases with ulnar nerve axonotmesis and available outcome data for intrinsic hand function were analysed to identify poor prognostic indicators. Demographic and injury features for those with 'poor' outcomes were compared against those with 'good' outcomes, and a paired t-test was applied to calculate statistical significance [Table - 10]. Older age and lower BMI were found to be statistically significant predictors of poor intrinsic hand recovery. Gender did not appear to be an indicator of intrinsic hand recovery; both outcome groups had an identical distribution of females. Vascular injuries and fractures were more frequent in those patients who regained good function. Rotator cuff tears were more frequent in the poor outcome group. Although these differences were not statistically significant, they demonstrate trends that can be acknowledged in clinical practice.

Patterns of infraclavicular brachial plexus injury without medial cord injury
The 10 cases without damage to the medial cord were reviewed [Figure - 6]. Some 30% of cases were found to have three cords intact; these injuries were only neurapraxic in nature. Moreover, 40% of cases had intact medial and posterior cords; no cases had an intact lateral cord.
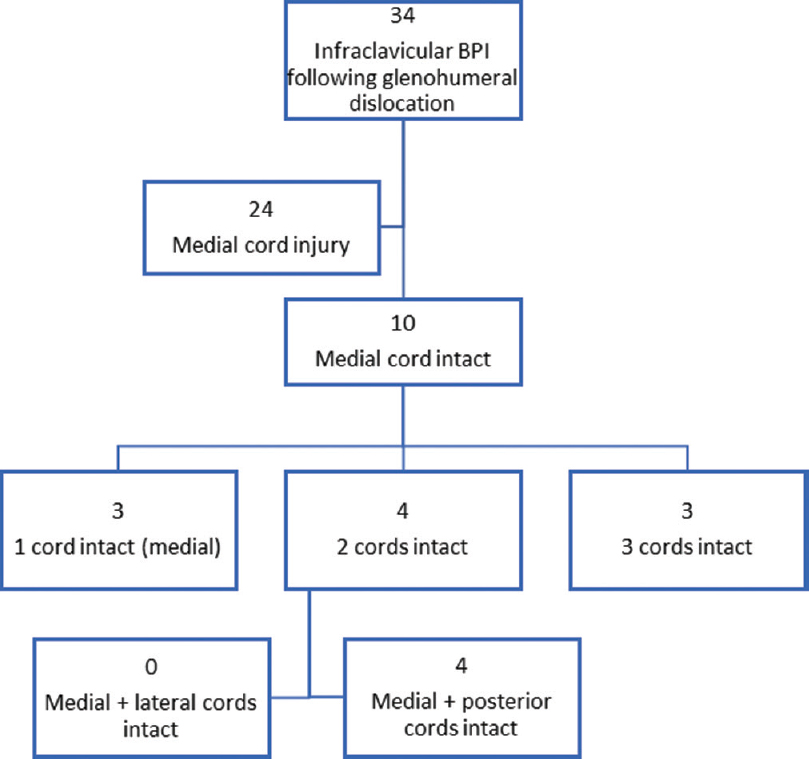 |
| Figure 6: Injury patterns in 10 patients with an intact medial cord following glenohumeral dislocation |
Discussion
There is limited published evidence on the patterns of injury, management and outcomes for infraclavicular BPIs. Medial cord involvement is common, and outcomes for grip and dextrous hand function are uncertain. High injuries to the medial cord are complex due to the long reinnervation distances required to reach distal motor targets and the limited window of time before motor endplates degenerate and muscles atrophy. This renders outcome from low-grade axonotmesis injuries unpredictable, with some patients regaining finger flexion but few regaining intrinsic function unless the initial injury is mixed with some preservation of motor axons to the intrinsic muscle across the site of injury.
Currently, the reconstructive options for these patients are limited to arthrodesis and tendon transfers when the neurological recovery has plateaued at 18–24 months following injury. Nerve transfers have become mainstream management strategies in supraclavicular injuries to the brachial plexus but are not routinely utilised in infraclavicular injuries due to the multiple nerve involvement and typically long reinnervation distances. Novel-staged nerve transfer techniques using less injured expendable branches within other cords may provide a solution to this problem and must be evaluated through clinical trials. The first stage of retrograde reinnervation of a cutaneous nerve needs to be performed early. The second stage distal reflection of the newly innervated nerve to a denervated target provides a possibility for motor recovery if and when spontaneous reinnervation through the medial cord has failed. A greater understanding of injury severity and predictors of recovery are therefore required at first presentation for guiding subsequent management.
In this case series, 34% of infraclavicular BPIs were attributed to glenohumeral dislocation sustained as a result of a low-energy fall. The majority (71%) had medial cord axonotmesis. More severe injuries presented in patients who were female, aged over 80 years and with a BMI exceeding 25. Moreover, older age and lower BMI were found to be statistically significant predictors of poor intrinsic hand recovery in those with ulnar nerve axonotmesis. Older age is likely to be associated with poor nerve recovery for a number of established reasons. First, the amount of myelin decreases with age as a result of diminished Schwann cell responses; therefore, nerve repair and regeneration are less efficient. Other important changes likely to hamper recovery in older individuals are reduced muscle strength, endoneurial blood flow and axonal sprouting. The association with BMI is slightly contradictory; while those with higher BMIs sustained more severe nerve injuries, they were more likely to recover a better grade of power. This perhaps suggests a supportive role of adipose tissue in nerve regeneration; indeed, adipose-derived stem cells are known to secrete growth factors that promote axonal growth, as well as being capable of differentiating into Schwann cells.[12]
Limitations
First, numbers are relatively small. This limits generalisability. However, these injuries are uncommon, so the findings still carry weight perhaps a multicentre collaboration with similar peripheral nerve services is warranted to increase significance. Furthermore, patient records were often incomplete or inconsistent, further compounding the small study population. In an effort to minimise these limitations for future data collection, we have developed a pro forma that will be implemented at the peripheral nerve service to make assessment of BPIs more systematic and datasets more uniform. This will hopefully allow early recognition of an injury subgroup with medial cord damage and concomitant sparing of the posterior and/or lateral cords which may provide an opportunity for consideration of novel-targeted nerve transfer surgery.
Conclusion
Early recognition of an injury subgroup with medial cord involvement and concomitant sparing of the posterior and/or lateral cords may provide an opportunity for consideration of novel-targeted nerve transfer surgery. This can restore hand function despite the long reinnervation distances necessary to reach forearm and hand motor targets. Ultimately, the timing and effectiveness of surgical interventions for infraclavicular BPIs will depend on the mechanism, pattern and grade of nerve injury. Clinical examination and electrodiagnosis are invaluable tools to diagnose these injuries. Other prognostic factors such as associated vascular, bone and soft tissue injuries, as well as patient demographics must also be taken into account to provide an accurate prognosis and determine amenability to these novel nerve transfers.
Ethical approval
This research audit was registered on the University Hospitals Birmingham NHS Foundation Trust's Clinical Audit Registration and Management System (CARMS). Ethical approval was obtained as appropriate.
Acknowledgement
We thank our colleagues from the Peripheral Nerve Service at University Hospitals Birmingham NHS Trust and the HaPPeN (Hands, Plastics and Peripheral Nerve Research Network) team for their contributions and expertise that greatly assisted this research.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Author's contributions
DMP conceived the study, which was designed by IAG, CJE and DNG. IAG and DNG collected and analysed data. IAG interpreted the data and wrote the paper; all other authors provided logistical support as required. All authors have critically reviewed and approved the paper, and are responsible for the content and similarity index of the manuscript.
| 1. | Leffert RD, Seddon H. Infraclavicular brachial plexus injuries. J Bone Joint Surg Br 1965;47:9-22. [Google Scholar] |
| 2. | Woo A, Bakri K, Moran SL. Management of ulnar nerve injuries. J Hand Surg Am 2015;40:173-81. [Google Scholar] |
| 3. | Delbe P, Cauchoix A. Paralysis in shoulder dislocations. Rev Chirurgie 1910;41:327-52. [Google Scholar] |
| 4. | Brown JM, Mackinnon SE. Nerve transfers in the forearm and hand. Hand Clin 2008;24:319-40, v. [Google Scholar] |
| 5. | Mackinnon S, Dellon A. Surgery of the Peripheral Nerve. 1st ed. New York: Thieme Medical Publishers; 1988. [Google Scholar] |
| 6. | Gutkowska O, Martynkiewicz J, Mizia S, Bąk M, Gosk J. Results of operative treatment of brachial plexus injury resulting from shoulder dislocation: A study with A long-term follow-up. World Neurosurg 2017;105:623-31. [Google Scholar] |
| 7. | Hems TE, Mahmood F. Injuries of the terminal branches of the infraclavicular brachial plexus: Patterns of injury, management and outcome. J Bone Joint Surg Br 2012;94:799-804. [Google Scholar] |
| 8. | Bruyns CN, Jaquet JB, Schreuders TA, Kalmijn S, Kuypers PD, Hovius SE, et al. Predictors for return to work in patients with median and ulnar nerve injuries. J Hand Surg Am 2003;28:28-34. [Google Scholar] |
| 9. | Hayter C. Overview of the UK Population. Ons.gov.uk; 2017. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/articles/overviewoftheukpopulation/july2017#toc. [Last accessed on 2018 Mar 14]. [Google Scholar] |
| 10. | de Laat EA, Visser CP, Coene LN, Pahlplatz PV, Tavy DL. Nerve lesions in primary shoulder dislocations and humeral neck fractures. A prospective clinical and EMG study. J Bone Joint Surg Br 1994;76:381-3. [Google Scholar] |
| 11. | Belzberg AJ, Dorsi MJ, Storm PB, Moriarity JL. Surgical repair of brachial plexus injury: A multinational survey of experienced peripheral nerve surgeons. J Neurosurg 2004;101:365-76. [Google Scholar] |
| 12. | Allbright KO, Bliley JM, Havis E, Kim DY, Dibernardo GA, Grybowski D, et al. Delivery of adipose-derived stem cells in poloxamer hydrogel improves peripheral nerve regeneration. Muscle Nerve 2018;58:251-60. [Google Scholar] |
Fulltext Views
8,161
PDF downloads
2,291





