Translate this page into:
Should bone densitometry define osteoporosis in 2020? A current concepts review of the role of vibrational spectroscopy in the evaluation of bone health
2 Department of Orthopedics, Shifa Hospital, Islamabad, Pakistan
3 College of Medicine, Taibah University, Almadinah Almunawwarah, Saudi Arabia
4 College of Medicine in Hradee Kralove, Charles University, Czech Republic
Corresponding Author:
Mohamed Khalid
Department of Orthopedics, College of Medicine, Taibah University, Prince Naif Road, Almadinah Almunawwarah
Saudi Arabia
movingbones1@gmail.com
| How to cite this article: Khalid M, Khan F, Baik MS, Fazal J. Should bone densitometry define osteoporosis in 2020? A current concepts review of the role of vibrational spectroscopy in the evaluation of bone health. J Musculoskelet Surg Res 2020;4:118-123 |
Abstract
Bone mineral density (BMD) is the most widely used parameter for measuring bone strength. Indeed, the World Health Organization definition of osteoporosis is based solely on the BMD as measured by dual energy X-ray absorptiometry (DEXA). As our understanding of the factors contributing to bone strength has improved in recent years, this might need to be re-visited. In this review, we have outlined the recent advances in our understanding of the structural health of the bone, specifically how whole bone geometry, micro-architecture and tissue properties are all factors that determine bone strength. We have outlined the importance of micro-crack formation and the pathways that could result following micro-crack formation. We have also presented evidence that makes a case for seeking an alternative technique to DEXA that could potentially improve/augment our ability to assess osteoporosis. Vibrational spectroscopic techniques such as Raman spectroscopy and Fourier transform infrared spectroscopy are evolving as important modalities that have the capability to evaluate all the determinants of bone strength qualitatively and quantitatively in a spatially resolved manner that could potentially provide a much more accurate assessment of bone health.
Introduction
Osteoporosis is defined by the National Osteoporosis Foundation as a chronic, progressive disease characterized by low bone mass, microarchitecture deterioration of bone tissue, bone fragility, and a consequent increase in fracture risk.[1] Although this comprehensive definition includes microarchitecture deterioration, the operational definition of osteoporosis is based entirely on the bone mineral density (BMD) measurement. The World Health Organization (WHO) in 1994, defined osteoporosis as a BMD that lies 2.5 standard deviations (SDs) or more below the average value for young healthy women (a T-score of <−2.5 SD).[2] The most widely used method to measure the BMD is dual energy X-ray absorptiometry (DEXA). Its advantages include low cost, low radiation, and patient convenience. Subsequently, it became increasingly obvious that a majority of fragility fractures indeed occurred in individuals whose BMD was not below the osteoporotic threshold of a T-score <−2.5 SD.[3],[4],[5],[6],[7] In the 25 years, since the WHO definition was proposed and widely adapted, our understanding of the bone micro-structure and how it deteriorates in osteoporosis, as well as the underlying pathological process involved, has progressed considerably. Our ability to detect these changes has also evolved. This review aims to highlight these advances and let the reader answer the question posed in the title.
Bio-Mechanical Considerations
Loading of Bone Results in Stress. The bone responds by dissipating the stress as well as by resisting deformation up to a certain limit known as the yield point. Beyond the yield point, strain starts to develop and could ultimately result in structural failure. The maximal load that can be applied to the bone before structural failure occurs is defined as the strength of the bone. The factors that determine the bone strength are illustrated in [Figure - 1] and are discussed below.
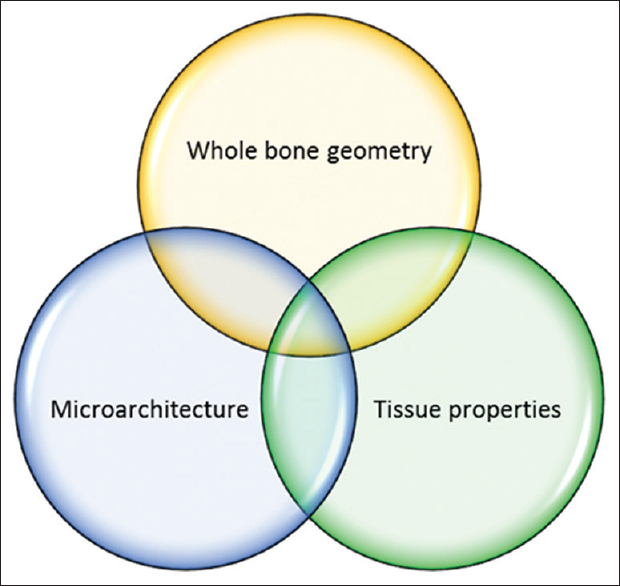 |
| Figure 1: Determinants of bone strength |
The mechanical performance of a composite structure like bone is not only reliant on the material properties of its components but also on the manner in which the material is laid out, or in other words, its architecture. The architecture could be conceptualized as macro-architecture or whole bone geometry and the microarchitecture. As the bone is a living tissue, the biological properties determine the dynamic responses to mechanical challenges and thus are central to the viability of the bone. Thus, it is important to consider all the above determinants of bone strength in order to assess the mechanical performance of the bone in health and disease.
Determinants of Bone Strength
Whole bone geometry
The bone geometry contributes to bone strength,[8] and fragility fractures.[9] An increase in bone diameter leads to an exponential increase in resistance to bending and torsion independent of bone mass.[10] Studies have shown that differences in the shape of the proximal femora, such as an increase in the length of the femoral neck and neck-shaft angle, are independent variables associated with an increased risk of sustaining a femoral neck fracture.[11],[12],[13] Increased length of the femoral neck leads to an increased moment resulting in a higher concentration of forces in the femoral neck if the person falls sideways.[14] Increased cross-sectional area (CSA) is associated with an increased bone strength index independent of BMD.[15] Indeed, it has been shown that differences in the bone strength between African and Caucasian postmenopausal women[16],[17] as well as elderly men and women[18],[19] are attributable to differences in the CSA of the bone. Further, cortical thickness but not cortical BMD was found to correlate significantly with the risk of developing fractures.[20]
Microarchitecture
Microarchitectural deterioration of cortical as well as trabecular bone leads to a significant diminution in the bone strength. Due to a larger total surface area relative to volume, trabecular bone is predominantly affected when there is increased bone resorption in osteoporosis. Trabecular microarchitecture is known to vary within the same bone and has been shown to be more relevant than BMD with respect to the site of fracture and the load to failure during compression testing.[21] Trabecular shape (plate-like or rod-like) and thickness significantly influences bone strength, and constitute an independent variable that determines bone strength.[22] Loss of continuity of the trabeculae results from the perforation of individual trabeculae. These changes characterize microarchitecture deterioration.[23],[24],[25] Regions such as long-bone metaphyses, and vertebral bodies that have a higher proportion of cancellous bone are affected disproportionately in this process,[26],[27] leading to bone fragility in these areas. Females predominantly show a decrease in trabecular number and hence a corresponding increase in trabecular separation, while in the case of males, the prominent feature is a decrease in trabecular thickness.[28] In areas such as the shaft of long bones where cortical bone constitutes a major proportion of the total bone mass, structural deterioration of the cortical bone, if present, significantly contributes to bone fragility. Osteoclasts present in the Haversian channel network, when activated, can cause widening of the channels and hence increased porosity resulting in loss of bone strength.[29],[30] An increase in cortical porosity has been shown to be associated with aging independent of the BMD.[31]
Tissue properties
The bone tissue is a two-phase composite with an elastic component mainly composed of type I collagen and a mineral component largely in the form of hydroxyapatite. Type I collagen is produced by osteoblasts initially as a precursor molecule procollagen that subsequently undergoes Post-translational modification in the extracellular matrix and formation of intermolecular and intra-fibrillar cross-links[32] that maintain the closely organized fibrillar structure of collagen and contribute to its tensile strength. The newly formed collagen molecule provides a platform for initial mineralization (primary mineralization), which gradually progresses in terms of the number and size of the crystals (secondary mineralization).[33]
The bone tissue properties and their relevance in osteoporosis can be best understood in the context of the response of bone tissue to loading and microcrack formation/propagation [Figure - 2]. Microcrack formation is known to trigger osteocyte apoptosis,[34],[35] and the resultant relaxation of the inhibitory control over the osteoclasts[36] as well as the release of stimulatory factors as well as a release of inhibition which in turn leads to osteoclast activation by a combination of loss of constitutive inhibition of osteocytes over osteoclasts and from the release of stimulatory substances.[34],[37] These events result in an increase in remodeling locally.[38] In addition to the above, osteocytes also recruit osteoblasts in response to loading by sensing the hydrostatic pressure of the interstitial fluid as well as detecting cell strain.[39],[40] And by chemical signaling involving nitric oxide,[41] Prostaglandins,[42],[43] and Sclerostin[44] that modulate osteoblast activity. Beyond a certain capacity to withstand the strain, the bone starts failing structurally, initially by the microcrack formation and if the amount of load keeps increasing, and there is insufficient capacity or time to repair the microcracks, by the propagation of the microcracks and resulting catastrophic failure, which clinically manifests as a fracture.
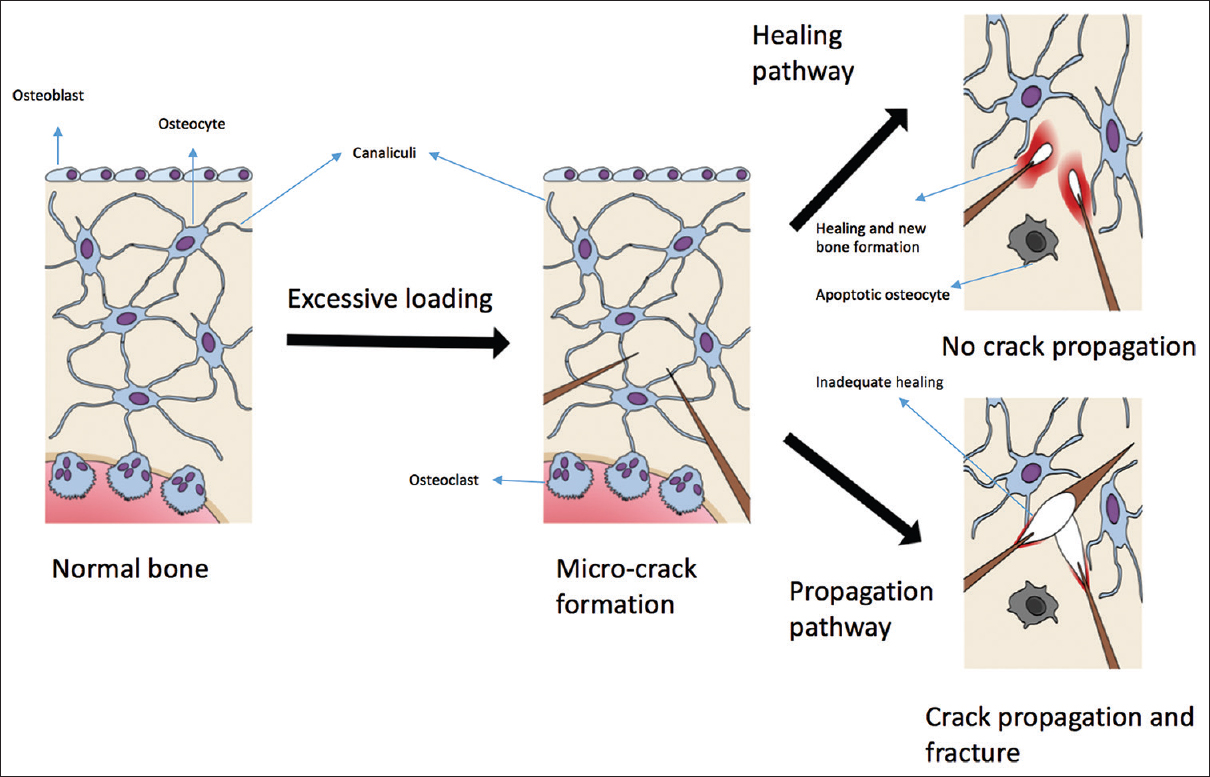 |
| Figure 2: Excessive loading leads to microcrack formation. Following microcrack formation, there are two possible pathways, healing, or propagation. The healing pathway leads to new bone formation and the stoppage of the microcrack. The propagation pathway leads to widening and fracture |
It is obvious from the foregoing account of tissue properties that they play an important role in the way bone reacts to loads, normal and abnormal. It is also obvious that bulk properties such as the bone mass or bone density do not represent the complete picture in terms of the structural strength and performance of the bone. Consequently, investigations such as DEXA or even quantitative computed tomography (qCT) are not reliable diagnostic or prognostic indicators. This is borne out by several studies, for example, the reduction in bone strength associated with aging is much steeper than the corresponding reduction in BMD.[45] A significant increase in BMD as a result of sodium fluoride therapy was shown to result in an increased rather than a decreased incidence of fragility fractures.[46],[47] Improvements in bone strength following exercise have been shown to be independent of BMD in several studies.[48],[49],[50],[51] Thus, there is a need to explore a diagnostic modality that could provide more information about the determinants of bone tissue properties in order to diagnose osteoporosis. If it is shown to provide a more accurate assessment of the structural strength of the bone, before gross bone destruction takes place, it could pave the way for early diagnosis and hence, better preventive/regenerative strategies to be adapted. Vibrational spectroscopic techniques such as Raman spectroscopy (RS) and Fourier transform infrared spectroscopy (FTIR) are very promising techniques that deserve emphasis. Their ability to capture tissue heterogeneity at a microscopic level is very helpful because it is well known that even within the same bone, there is a mosaic of pockets of bone resorption and bone formation as well as dormant areas and a balance between them often dictates the mechanical performance of the bone.[52]
Vibrational spectroscopic techniques
Both Raman and FTIR spectroscopy depend on the transition of vibrational energy states of molecules. Infrared spectra are derived directly from the absorption of energy in the infrared range, whereas Raman spectra arise from a scattering of visible or ultra-violet photons. In addition, the biomolecular milieu surrounding the molecule of interest also influence the vibrational pattern and hence can also be analyzed.[53] The pattern of an individual vibrational band is unique for a particular functional group or molecular species.[54] The Vibrational spectral patterns are, for the most part, wavelength-specific; thus, a combination of Raman and FTIR can provide complementary information regarding the test sample [Figure - 3].[55] We have previously studied changes in mineralization and collagen structure in human bone using RS.[56] Water does not interfere with this technique and hence it could potentially be used to monitor tissues in vivo, noninvasively. The other major advantage is that the tissues can be studied in their native state without any preparations such as contrast media or dyes.
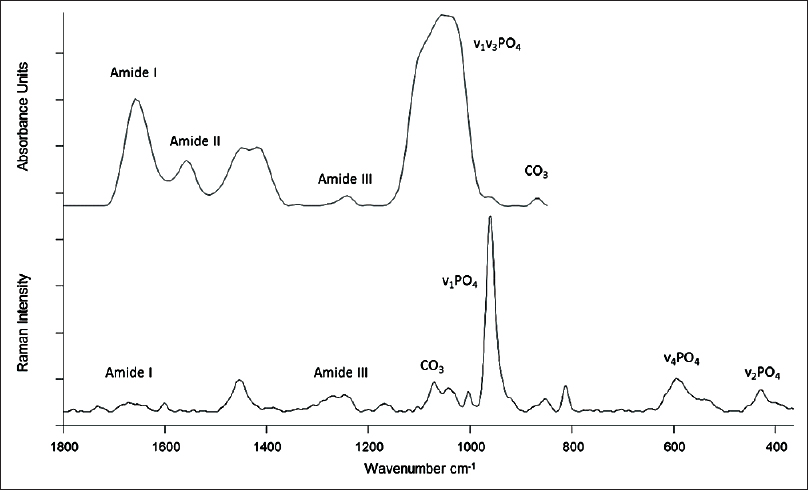 |
| Figure 3: Typical Fourier transform infrared spectroscopy (top) and Raman spectra (bottom) with the commonly studied peaks appropriately marked. Wavenumber is the typical unit of frequency used in vibrational spectroscopy measured in reciprocal centimeters |
The most useful and commonly assessed spectroscopic parameters are the following:
- Mineral to matrix ratio: This is the most commonly assessed parameter and has been validated against mineral content measurement by quantitative back-scatter electronic imaging in human bones.[57] It is based on the principle that the integrated area of a band is directly proportional to the concentration of the specific molecular moiety giving rise to it. Most commonly, the ratio of phosphate to amide bands is measured. This is a spectroscopic equivalent of BMD with the added benefit of providing information on the organic matrix (collagen) in a spatially distributed manner
- Mineral maturity/crystallinity: The chemical makeup of the mineral crystals and their similarity to pure hydroxyapatite crystals is known as crystal maturity whereas the size and the shape of the crystals are referred to as crystallinity. This parameter is highly dependent on the person's age as well as the tissue age in the same person. Heterogeneity of crystals is seen in young healthy bone[58] whereas homogeneity as well as the presence of large crystals is often associated with ageing[59] and osteoporosis[60]
- Carbonate to phosphate ratio: Bone mineral consists of highly substituted apatite crystals. Carbonate is one of the most abundant substitutions. In healthy bone, the average carbonate is about 6% dry weight.[61] It is most commonly reported as carbonate to phosphate ratio although some report it as carbonate to organic matrix ratio. Carbonate content is known to be altered in osteoporosis[62],[63]
- Relative tissue water content: The contribution of water to the biomechanical properties of the bone is well established.[64],[65] Water can be directly measured through quantification of hydroxyl groups by RS[66]
- Collagen cross-linking: About 90% of the entire organic component of the bone is composed of type I collagen which is a large fibrous protein made up of a triple helix (two α1 and one α2 chains). The most distinctive feature of mineralizing collagen in bone is its cross-linking chemistry and the way the molecule is packed,[67] and contributes significantly to the mechanical properties such as tensile strength and viscoelasticity. The analysis of amide I band can be used to study pyridinoline cross-links and is the parameter most often used to study the cross-link chemistry.[68] It is also the most sensitive spectrometric parameter that differentiates ageing from osteoporotic bone[69]
- Relative proteoglycan content: Proteoglycans are large noncollagenous glycoproteins. In the bone, they fulfill several important roles such as organic matrix assembly and modulation of mineralization as well as remodeling.[70] They also help maintain unhindered flow of the interstitial fluid through the peri-lacunar/canalicular space of the compact bone by preventing its mineralization.[71] RS can be used to study glycosaminoglycan component of proteoglycans in the bone[72] and a decrease of the same has been reported in postmenopausal osteoporosis.[69]
Several challenges have to be overcome in order to make these techniques widely acceptable as useful clinical tools. With RS, the main challenges have been (a) low signal to noise ratio, (b) background fluorescence, and (c) difficulty in assessing deeper structures as it is largely a surface analytic technique. Various technological advances have largely overcome the signal to noise ratio problem.[73] Background fluorescence problem can be minimized by having a long excitation wavelength[74] and using data evaluation techniques such as Band Target Entropy Minimization technique.[75] Spatially off-set RS,[76] and picosecond time-resolved spectroscopy[77] have made it possible to use this technique for analysis of deeper structures in vivo. The main drawback of FTIR is that it is, as yet, anin vitro technique and thus, invasive biopsies are required. These biopsies can be obtained if/when the patient is undergoing a bony surgical procedure such as open reduction and internal fixation of a fracture or arthroplasty. Data obtained from the FTIR could provide useful complementary information which can be extrapolated to Raman data as spectroscopic theory allows this extrapolation.[53] Thus, the usefulness of RS as a clinical investigative tool can be enhanced.
In conclusion, these vibrational spectroscopic techniques could potentially facilitate early diagnosis of structural deterioration of the bone at the tissue level. RS has the potential to do this noninvasively and FTIR in a minimally invasive way, before gross/irreversible structural changes occur in the bone architecture.
The ability of vibrational spectroscopic techniques to assess the collagen as well the mineral components of the bone and their intricate inter-relationship and to do so in a spatially distributed manner give these techniques a decisive advantage over DEXA scanning. Drawbacks such as poor signal to noise ratio, need to obtain a biopsy, and portability of the equipment have been largely overcome and the time is ripe for vibrational spectroscopic techniques to move from the bench to the clinic. We recommend that large scale clinical trials evaluating the usefulness of these techniques vis-à-vis established techniques such as DEXA and qCT be conducted. Such efforts would conceivably go a long way toward assessment and monitoring of bone health.
Ethical consideration
Ethics: As this is a review no ethical committee permission was obtained.
Financial support and sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
There are no conflicts of interest.
Authors' contribution
MK conceived the project and was involved with the literature review and writing the manuscript. FK helped with the writing of the manuscript and formatting the references. MSB and JF were involved with the literature search and helped write the manuscript. All authors have reviewed the manuscript, agree with the contents, and take full responsibility for the accuracy of the information contained.
| 1. | Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int 2014;25:2359-81. [Google Scholar] |
| 2. | Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis. Report of a WHO Study Group (WHO Technical Report Series, No. 843). Geneva: World Health Organization; 1994. [Google Scholar] |
| 3. | Schuit SC, Van der Klift M, Weel AE, De Laet CE, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: The Rotterdam study. Bone 2004;34:195-202. [Google Scholar] |
| 4. | Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD. Low bone mineral density and fracture burden in postmenopausal women. CMAJ 2007;177:575-80. [Google Scholar] |
| 5. | Pasco JA, Seeman E, Henry MJ, Merriman EN, Nicholson GC, Kotowicz MA. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int 2006;17:1404-9. [Google Scholar] |
| 6. | Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, et al. BMD at multiple sites and risk of fracture of multiple types: Long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 2003;18:1947-54. [Google Scholar] |
| 7. | Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM, Hillier TA, et al. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab 2005;90:2787-93. [Google Scholar] |
| 8. | Fonseca H, Moreira-Gonçalves D, Vaz M, Fernandes MH, Ferreira R, Amado F, et al. Changes in proximal femur bone properties following ovariectomy and their association with resistance to fracture. J Bone Miner Metab 2012;30:281-92. [Google Scholar] |
| 9. | Alele JD, Kamen DL, Hunt KJ, Ramsey-Goldman R. Bone geometry profiles in women with and without SLE. J Bone Miner Res 2011;26:2719-26. [Google Scholar] |
| 10. | Turner CH, Burr DB. Basic biomechanical measurements of bone: A tutorial. Bone 1993;14:595-608. [Google Scholar] |
| 11. | El-Kaissi S, Pasco JA, Henry MJ, Panahi S, Nicholson JG, Nicholson GC, et al. Femoral neck geometry and hip fracture risk: The Geelong osteoporosis study. Osteoporos Int 2005;16:1299-303. [Google Scholar] |
| 12. | Frisoli A Jr., Paula AP, Pinheiro M, Szejnfeld VL, Delmonte Piovezan R, Takata E, et al. Hip axis length as an independent risk factor for hip fracture independently of femural bone mineral density in Caucasian elderly Brazilian women. Bone 2005;37:871-5. [Google Scholar] |
| 13. | Bergot C, Bousson V, Meunier A, Szejnfeld VL, Delmonte Piovezan R, Takata E, et al. Hip fracture risk and proximal femur geometry from DXA scans. Osteoporos Int 2002;13:542-50. [Google Scholar] |
| 14. | Wang Q, Teo JW, Ghasem-Zadeh A, Seeman E. Women and men with hip fractures have a longer femoral neck moment arm and greater impact load in a sideways fall. Osteoporos Int 2009;20:1151-6. [Google Scholar] |
| 15. | Duncan CS, Blimkie CJ, Kemp A, Higgs W, Cowell CT, Woodhead H, et al. Mid-femur geometry and biomechanical properties in 15- to 18-yr-old female athletes. Med Sci Sports Exerc 2002;34:673-81. [Google Scholar] |
| 16. | Nelson DA, Pettifor JM, Barondess DA, Cody DD, Uusi-Rasi K, Beck TJ. Comparison of cross-sectional geometry of the proximal femur in white and black women from Detroit and Johannesburg. J Bone Miner Res 2004;19:560-5. [Google Scholar] |
| 17. | Nelson DA, Barondess DA, Hendrix SL, Beck TJ. Cross-sectional geometry, bone strength, and bone mass in the proximal femur in black and white postmenopausal women. J Bone Miner Res 2000;15:1992-7. [Google Scholar] |
| 18. | Russo CR, Lauretani F, Bandinelli S, Bartali B, Di Iorio A, Volpato S, et al. Aging bone in men and women: Beyond changes in bone mineral density. Osteoporos Int 2003;14:531-8. [Google Scholar] |
| 19. | Russo CR, Lauretani F, Seeman E, Bartali B, Bandinelli S, Di Iorio A, et al. Structural adaptations to bone loss in aging men and women. Bone 2006;38:112-8. [Google Scholar] |
| 20. | Taes Y, Lapauw B, Griet V, De Bacquer D, Goemaere S, Zmierczak H, et al. Prevalent fractures are related to cortical bone geometry in young healthy men at age of peak bone mass. J Bone Miner Res 2010;25:1433-40. [Google Scholar] |
| 21. | Legrand E, Chappard D, Pascaretti C, Duquenne M, Krebs S, Rohmer V, et al. Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J Bone Miner Res 2000;15:13-9. [Google Scholar] |
| 22. | Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, et al. Effects of sex and age on bone microstructure at the ultradistal radius: A population-based noninvasivein vivo assessment. J Bone Miner Res 2006;21:124-31. [Google Scholar] |
| 23. | Sran MM, Boyd SK, Cooper DM, Khan KM, Zernicke RF, Oxland TR. Regional trabecular morphology assessed by micro-CT is correlated with failure of aged thoracic vertebrae under a posteroanterior load and may determine the site of fracture. Bone 2007;40:751-7. [Google Scholar] |
| 24. | Siu WS, Qin L, Cheung WH, Leung KS. A study of trabecular bones in ovariectomized goats with micro-computed tomography and peripheral quantitative computed tomography. Bone 2004;35:21-6. [Google Scholar] |
| 25. | Laib A, Kumer JL, Majumdar S, Lane NE. The temporal changes of trabecular architecture in ovariectomized rats assessed by MicroCT. Osteoporos Int 2001;12:936-41. [Google Scholar] |
| 26. | Boyd SK, Davison P, Müller R, Gasser JA. Monitoring individual morphological changes over time in ovariectomized rats byin vivo micro-computed tomography. Bone 2006;39:854-62. [Google Scholar] |
| 27. | Turner CH. Biomechanics of bone: Determinants of skeletal fragility and bone quality. Osteoporos Int 2002;13:97-104. [Google Scholar] |
| 28. | Ikeda S, Tsurukami H, Ito M, Sakai A, Sakata T, Nishida S, et al. Effect of trabecular bone contour on ultimate strength of lumbar vertebra after bilateral ovariectomy in rats. Bone 2001;28:625-33. [Google Scholar] |
| 29. | Cooper DM, Thomas CD, Clement JG, Turinsky AL, Sensen CW, Hallgrímsson B, et al. Age-dependent change in the 3D structure of cortical porosity at the human femoral mid-shaft. Bone 2007;40:957-65. [Google Scholar] |
| 30. | Sietsema WK. Animal models of cortical porosity. Bone 1995;17:297S-305. [Google Scholar] |
| 31. | Nicks KM, Amin S, Atkinson EJ, Riggs BL, Melton LJ III, Khosla S. Relationship of age to bone microstructure independent of areal bone mineral density. J Bone Miner Res 2012;27:637-44. [Google Scholar] |
| 32. | Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int 2006;17:319-36. [Google Scholar] |
| 33. | Golub EE. Role of matrix vesicles in biomineralization. Biochim Biophys Acta 2009;1790:1592-8. [Google Scholar] |
| 34. | Kurata K, Heino TJ, Higaki H, Väänänen HK. Bone marrow cell differentiation induced by mechanically damaged osteocytes in 3D gel-embedded culture. J Bone Miner Res 2006;21:616-25. [Google Scholar] |
| 35. | Eberhardt AW, Yeager-Jones A, Blair HC. Regional trabecular bone matrix degeneration and osteocyte death in femora of glucocorticoid- treated rabbits. Endocrinology 2001;142:1333-40. [Google Scholar] |
| 36. | Gu G, Mulari M, Peng Z, Hentunen TA, Väänänen HK. Death of osteocytes turns off the inhibition of osteoclasts and triggers local bone resorption. Biochem Biophys Res Commun 2005;335:1095-101. [Google Scholar] |
| 37. | Kogianni G, Mann V, Noble BS. Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localized bone destruction. J Bone Miner Res 2008;23:915-27. [Google Scholar] |
| 38. | Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res 2009;24:597-605. [Google Scholar] |
| 39. | Liu C, Zhao Y, Cheung WY, Gandhi R, Wang L, You L. Effects of cyclic hydraulic pressure on osteocytes. Bone 2010;46:1449-56. [Google Scholar] |
| 40. | Bonivtch AR, Bonewald LF, Nicolella DP. Tissue strain amplification at the osteocyte lacuna: A microstructural finite element analysis. J Biomech 2007;40:2199-206. [Google Scholar] |
| 41. | Vatsa A, Mizuno D, Smit TH, Schmidt CF, MacKintosh FC, Klein-Nulend J. Bio imaging of intracellular NO production in single bone cells after mechanical stimulation. J Bone Miner Res 2006;21:1722-8. [Google Scholar] |
| 42. | Ajubi NE, Klein-Nulend J, Alblas MJ, Burger EH, Nijweide PJ. Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes. Am J Physiol 1999;276:E171-8. [Google Scholar] |
| 43. | Bakker AD, Soejima K, Klein-Nulend J, Burger EH. The production of nitric oxide and prostaglandin E(2) by primary bone cells is shear stress dependent. J Biomech 2001;34:671-7. [Google Scholar] |
| 44. | Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 2005;19:1842-4. [Google Scholar] |
| 45. | Keaveny TM, Kopperdahl DL, Melton LJ III, Hoffmann PF, Amin S, Riggs BL, et al. Age-dependence of femoral strength in white women and men. J Bone Miner Res 2010;25:994-1001. [Google Scholar] |
| 46. | Riggs BL, Hodgson SF, O'Fallon WM, Chao EY, Wahner HW, Muhs JM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 1990;322:802-9. [Google Scholar] |
| 47. | Meunier PJ, Sebert JL, Reginster JY, Briancon D, Appelboom T, Netter P, et al. Fluoride salts are no better at preventing new vertebral fractures than calcium-vitamin D in postmenopausal osteoporosis: The FAVO Study. Osteoporos Int 1998;8:4-12. [Google Scholar] |
| 48. | Järvinen TL, Kannus P, Sievänen H, Jolma P, Heinonen A, Järvinen M. Randomized controlled study of effects of sudden impact loading on rat femur. J Bone Miner Res 1998;13:1475-82. [Google Scholar] |
| 49. | Huang TH, Chang FL, Lin SC, Liu SH, Hsieh SS, Yang RS. Endurance treadmill running training benefits the biomaterial quality of bone in growing male Wistar rats. J Bone Miner Metab 2008;26:350-7. [Google Scholar] |
| 50. | Lespessailles E, Jaffre C, Beaupied H, Nanyan P, Dolléans E, Benhamou CL, et al. Does exercise modify the effects of zoledronic acid on bone mass, microarchitecture, biomechanics, and turnover in ovariectomized rats? Calcif Tissue Int 2009;85:146-57. [Google Scholar] |
| 51. | Adami S, Gatti D, Braga V, Bianchini D, Rossini M. Site-specific effects of strength training on bone structure and geometry of ultradistal radius in postmenopausal women. J Bone Miner Res 1999;14:120-4. [Google Scholar] |
| 52. | Einhorn TA. The bone organ system: Form and function. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. New York: Academic; 1996. [Google Scholar] |
| 53. | Paschalis EP, Gamsjaeger S, Klaushofer K. Vibrational spectroscopic techniques to assess bone quality. Osteoporos Int 2017;28:2275-91. [Google Scholar] |
| 54. | Gamsjaeger S, Mendelsohn R, Boskey AL, Gourion-Arsiquaud S, Klaushofer K, Paschalis EP. Vibrational spectroscopic imaging for the evaluation of matrix and mineral chemistry. Curr Osteoporos Rep 2014;12:454-64. [Google Scholar] |
| 55. | Socrates G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts. Chichester: Wiley; 2001. [Google Scholar] |
| 56. | Khalid M, Bora T, Al Ghaithi A, Thukral S, Dutta J. Raman spectroscopy detects changes in bone mineral quality and collagen crosslinkage in staphylococcus infected human bone. Sci Rep 2018;8:9417. [Google Scholar] |
| 57. | Roschger A, Gamsjaeger S, Hofstetter B, Masic A, Blouin S, Messmer P, et al. Relationship between the v(2)PO(4)/amide III ratio assessed by Raman spectroscopy and the calcium content measured by quantitative backscattered electron microscopy in healthy human osteonal bone. J Biomed Opt 2014;19:065002. [Google Scholar] |
| 58. | Boskey A. Bone mineral crystal size. Osteoporos Int 2003;14 Suppl 5:S16-20, S20-1. [Google Scholar] |
| 59. | Yerramshetty JS, Lind C, Akkus O. The compositional and physicochemical homogeneity of male femoral cortex increases after the sixth decade. Bone 2006;39:1236-43. [Google Scholar] |
| 60. | Donnelly E, Meredith DS, Nguyen JT, Gladnick BP, Rebolledo BJ, Shaffer AD, et al. Reduced cortical bone compositional heterogeneity with bisphosphonate treatment in postmenopausal women with intertrochanteric and subtrochanteric fractures. J Bone Miner Res 2012;27:672-8. [Google Scholar] |
| 61. | Ou-Yang H, Paschalis EP, Mayo WE, Boskey AL, Mendelsohn R. Infrared microscopic imaging of bone: Spatial distribution of CO3(2-). J Bone Miner Res 2001;16:893-900. [Google Scholar] |
| 62. | Iwasaki Y, Kazama JJ, Yamato H, Fukagawa M. Changes in chemical composition of cortical bone associated with bone fragility in rat model with chronic kidney disease. Bone 2011;48:1260-7. [Google Scholar] |
| 63. | Duboeuf F, Burt-Pichat B, Farlay D, Suy P, Truy E, Boivin G. Bone quality and biomechanical function: A lesson from human ossicles. Bone 2015;73:105-10. [Google Scholar] |
| 64. | Granke M, Does MD, Nyman JS. The role of water compartments in the material properties of cortical bone. Calcif Tissue Int 2015;97:292-307. [Google Scholar] |
| 65. | Unal M, Akkus O. Raman spectral classification of mineral- and collagen-bound water's associations to elastic and post-yield mechanical properties of cortical bone. Bone 2015;81:315-26. [Google Scholar] |
| 66. | Unal M, Yang S, Akkus O. Molecular spectroscopic identification of the water compartments in bone. Bone 2014;67:228-36. [Google Scholar] |
| 67. | Robins SP. Biochemistry and functional significance of collagen cross-linking. Biochem Soc Trans 2007;35:849-52. [Google Scholar] |
| 68. | McNerny EM, Gong B, Morris MD, Kohn DH. Bone fracture toughness and strength correlate with collagen cross-link maturity in a dose-controlled lathyrism mouse model. J Bone Miner Res 2015;30:455-64. [Google Scholar] |
| 69. | Paschalis EP, Fratzl P, Gamsjaeger S, Hassler N, Brozek W, Eriksen EF. Aging versus postmenopausal osteoporosis: Bone composition and maturation kinetics at actively-forming trabecular surfaces of female subjects aged 1 to 84 years. J Bone Miner Res 2016;31:347-57. [Google Scholar] |
| 70. | Gualeni B, de Vernejoul MC, Marty-Morieux C, De Leonardis F, Franchi M, Monti L, et al. Alteration of proteoglycan sulfation affects bone growth and remodeling. Bone 2013;54:83-91. [Google Scholar] |
| 71. | Thompson WR, Modla S, Grindel BJ, Czymmek KJ, Kirn-Safran CB, Wang L, et al. Perlecan/Hspg2 deficiency alters the pericellular space of the lacuna-canalicular system surrounding osteocytic processes in cortical bone. J Bone Miner Res 2011;26:618-29. [Google Scholar] |
| 72. | Gamsjaeger S, Klaushofer K, Paschalis E. Raman analysis of proteoglycans simultaneously in bone and cartilage. J Raman Spectrosc 2014;45:794-800. [Google Scholar] |
| 73. | Sin SY, Widjaja E, Yu LE, Garland M. Application of FT-Raman and FTIR measurements using a novel spectral reconstruction algorithm. J Raman Spectrosc 2003;34:795-805. [Google Scholar] |
| 74. | Hamada K, Fujita K, Smith NI, Kobayashi M, Inouye Y, Kawata S. Raman microscopy for dynamic molecular imaging of living cells. J Biomed Optics 2008;13:044027. [Google Scholar] |
| 75. | Pettway GJ, Schneider A, Koh AJ, Widjaja E, Morris MD, Meganck JA, et al. Anabolic actions of PTH (1-34): Use of a novel tissue engineering model to investigate temporal effects on bone. Bone 2005;36:959-70. [Google Scholar] |
| 76. | Buckley K, Kerns J, Gikas PD, Birch H, Vinton J, Keen R, et al. Measurement of abnormal bone compositionin vivo using noninvasive Raman spectroscopy. IBMS Bone Key 2014;11:1-3. [Google Scholar] |
| 77. | Draper ER, Morris MD, Camacho NP, Matousek P, Towrie M, Parker AW, et al. Novel assessment of bone using time-resolved transcutaneous Raman spectroscopy. J Bone Miner Res 2005;20:1968-72. [Google Scholar] |
Fulltext Views
3,359
PDF downloads
1,576





