Translate this page into:
The future of injectable orthobiologic substances for knee osteoarthritis: Options beyond platelet-rich plasma
Corresponding Author:
Karan Jindal
Post Graduate Institute of Medical Education and Research, Chandigarh
India
karan.121@hotmail.com
| How to cite this article: Patel S, Jindal K, Dhillon MS. The future of injectable orthobiologic substances for knee osteoarthritis: Options beyond platelet-rich plasma. J Musculoskelet Surg Res 2020;4:173-181 |
Abstract
The management of young arthritic knee and early grades of osteoarthritis (OA) aims to reduce morbidity and improve the quality of life for which newer modalities are emerging. Orthobiologics have emerged as a viable alternative option as they promote tissue regeneration and could be potential disease-modifying agents. Platelet-rich plasma (PRP) has been promising and is among the frontline treatment options in early OA knee; newer orthobiologic research is progressing beyond this and newer products are being tried. Various combinations of PRP with carriers and growth factor extracts from PRP are some new developments. Additional options beyond PRP include autologous conditioned serum, alpha-2 macroglobulin, adipose tissue derivative, bone marrow aspirate concentrate, and gene therapy. This review aims to shed light on the current literature and future potential of the use of these intra-articular orthobiologics in the 21st century.
Introduction
Osteoarthritis (OA) is a chronic and debilitating joint disease that affects the articular cartilage and underlying bone. Advanced and severe grades of OA warrant the need for joint replacement. However, the management of young arthritic knee and early grades of OA has seen much research in the early 21st century, and newer emerging modalities are allowing efficient management in this subgroup of patients.[1] The goal of treatment in the young arthritic knee is to reduce morbidity and improve the quality of life; delaying the progression of OA is a bonus. Modern thinking is focusing on “knee preservation,” which is now emerging as a viable option for treatment.[2] The application of products from biological sources has been labeled as orthobiologics, and it has evolved significantly over the past 10 years.[3] The application of these products, though showing sufficient promise, needs thorough proper case identification, and thorough evaluation and identification of mechanical and biological factors contributing to the pathology. In the knee, mechanical factors found to be contributory need relevant management (corrective osteotomy for varus alignment, meniscal root repairs, and repair of ramp lesions). Nevertheless, the role of alteration of intra-articular biology is increasingly recognized, and various treatment options are currently practiced. Platelet-rich plasma (PRP) has emerged as the frontline option for the management of early OA knee,[4],[5],[6],[7] and the research in the past 10 years has been promising. The success seen with PRP use has pushed scientists to try even newer modalities such as specific growth factors, interleukin-1 receptor antagonist protein (IRAP),[8],[9] Alfa-2 macroglobulin (A2M),[10],[11] and several other peptides. Gene therapy, which is focused on more sustained delivery of growth factors, is already in Phase 2[12],[13],[14] and Phase 3 trials.[15] This article discusses the recent modifications and trends in PRP use and introduce various other orthobiologics that have evolved for the management of OA [Figure - 1].
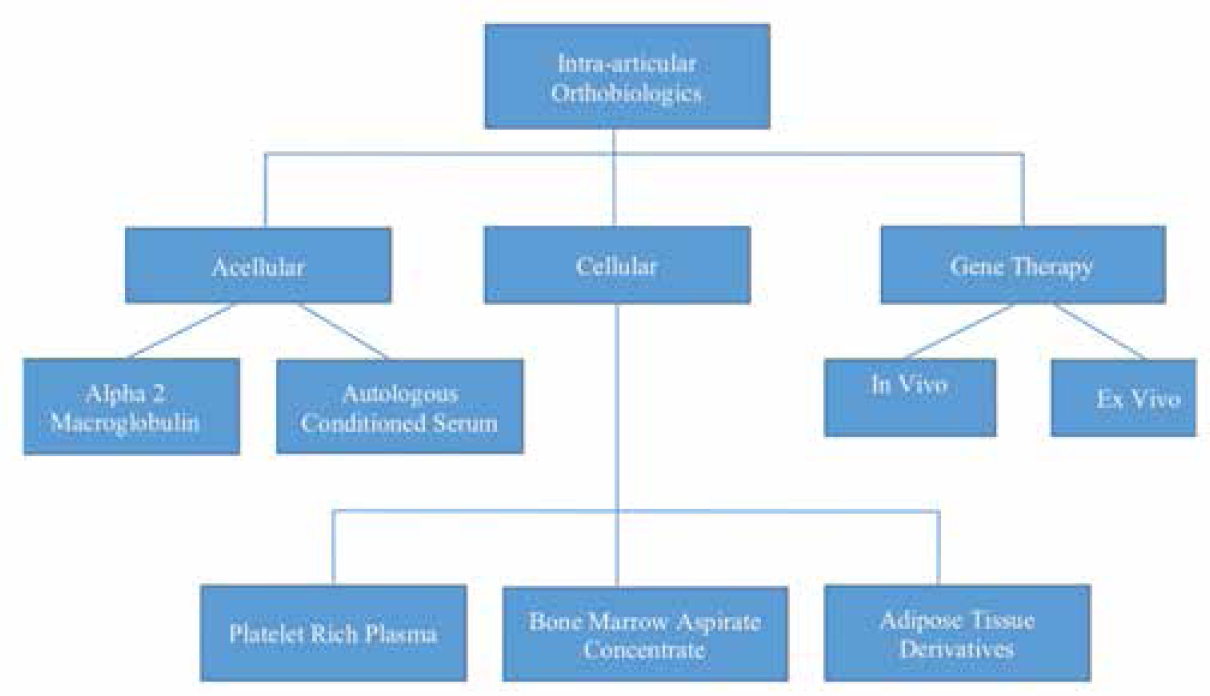 |
| Figure 1: Algorithm depicting intra-articular orthobiologics substances used for osteoarthritis knee in a simplified manner |
Role of Platelet-Rich Plasma in Knee Osteoarthritis
PRP, obtained by centrifugation of the patient's blood, contains biologically active proteins such as platelet-derived growth factor, tissue growth factor, fibroblast growth factor, and vascular endothelial growth factor, which reduce inflammation and cause cellular proliferation.[16] The role of PRP in alleviating pain and improving outcome scores has been established over the past decade.
Sánchez et al.,[17] in the first trial on PRP in 2008, showed that its intra-articular use is safe. Spaková et al.[18] established the safety and efficacy of PRP in early OA while comparing it with hyaluronic acid (HA) in a series of 120 patients. Patel et al.[4] showed its efficacy by comparing PRP with placebo (normal saline) and reported improved functional scores. This has been confirmed by many authors, and PRP is widely used as an intra-articular injection in the treatment of OA with excellent patient-reported outcomes.
Modifications in Platelet-Rich Plasma for Intra-Articular Use
Despite its frequent use, there is much variability in PRP use in terms of its formulation (leucocyte-rich PRP or leucocyte-poor PRP),[19],[20] use of activators,[21] number of injections administered,[22],[23] and the platelet concentration used.[24] The future research should ideally focus on answering these questions and thereby define the ideal PRP formulation and its dose for use in OA Knee.
Studies have shown that PRP combined with HA has a synergistic action by promoting cartilage regeneration and inhibition of inflammation as both target different biological pathways. Saturveithan et al.[25] in their randomized clinical trial (RCT) showed that combining PRP with HA significantly reduced pain and improved functional outcomes as compared to HA alone at 1 year of follow-up. However, there are reports of loss of rheological properties of HA due to dilution with PRP.[26] Low-molecular weight HA is better than high-molecular weight for combining with PRP and enhances the synergistic effects better.[26] Suboptimal results may occur by loss of viscoelastic properties due to dilution or lower concentration of HA. It, therefore, seems more logical for using a combination of PRP and HA by administering them separately with spaced duration rather than combining them together.[27] The ideal combination, ratio, and sequence needs to be evaluated and could be a future research topic.
There is an increasing focus on delivering PRP with carriers to improve and sustain the delivery of growth factors at the target site. Some of the potential carriers being tried are chitosan and gelatin hydrogel. Saito et al.,[28] in their study on rabbits, demonstrated that gelatin hydrogel PRP suppressed OA both histologically and morphologically to a greater extent as compared to PRP alone. Chitosan has shown some role in improving PRP's efficacy and causes better platelet adhesion and aggregation. Dwivedi et al.,[29] in an animal study involving 2 groups (PRP and freeze-dried chitosan with PRP), observed better scores with chitosan + PRP at 8 weeks. They hypothesized that in contrast to PRP, which is quickly degraded, chitosan PRP persisted for several weeksin vivo with longer-lasting effects.
Another approach being tried to improve PRP is the use of photoactivated PRP (PA-PRP). Photoactivation of peripheral blood improves the inflammatory mediators and synergistic action of PRP. Paterson et al.[30] conducted a double-blind randomized trial in 23 patients using intra-articular PA-PRP and HA. They reported significant improvement in symptoms and visual analog scale (VAS) score and knee injury and osteoarthritis outcome (KOOS) pain scores at 4 and 12 weeks with PA-PRP. However, no significant difference was found between the two groups (PA-PRP and HA) although PA-PRP did have improved symptoms and functional scores. Two patients had minor reactions of pain and swelling following PA-PRP. Despite promising results, there have not been any other trials reporting PA-PRP use in OA knees.
Another emerging concept is a combination of intraosseous and intra-articular injections of PRP for severe OA knee.[31] The rationale behind this is that OA also affects the underlying subchondral bone apart from changes in the articular cartilage and synovial fluid. Subchondral bone is becoming the potential therapeutic target in OA knee, and efforts are focusing on stimulating its remodeling. Sanchez et al.[32] conducted a pilot study in 13 patients and reported a substantial reduction in pain and improved functional scores. They later conducted a study in 60 patients in which they compared the combination therapy with PRP alone and reported that the combination of intra-articular PRP with intraosseous infiltration of PRP was clinically superior at 6 and 12 months.[33] Similarly, Su et al.[34] in a series of 86 patients compared 3 groups: combination (Intra-articular PRP + intraosseous PRP), intra-articular PRP, and intra-articular HA. They reported a significant improvement in VAS and Western Ontario and McMaster Universities (WOMAC) OA Index score at 18 months of intervention in the combined group. Thus, the use of intraosseous PRP is potentially emerging as a useful adjunct in the treatment of advanced OA.
Homologous PRP is obtained from healthy blood donors and has been assessed for patients with poor general health, who are not candidates for autologous PRP.[35] These include anemic patients, those with platelet dysfunction, or hematological disorders. Bottegoni et al.,[35] in their pilot study, used homologous PRP in 60 patients and reported an excellent safety profile, but only a short-term clinical improvement. Functional scores improved at 2 months and 6 months from baseline. However, they reported poor results in patients aged over 80 years or in severe OA. They concluded that this could be considered in patients who are not suitable for autologous PRP. The study, however, did not have any controls and no randomization was done.
Growth Factor Concentrate from Platelet-Rich Plasma
Another emerging concept is to extract the growth factors from PRP and inject the growth factor rich solution. This can be achieved using an activator to activate the PRP, which leads to degranulation and release of GFs, which can then be injected. The final products are acellular growth factor-rich concentrates.
This was first used and popularized by Anitua et al.[36] who used plasma rich in growth factors (PRGF-Endoret). They reported improvement in WOMAC and VAS scores as compared to HA. Vaquerizo et al.[37] showed improved results with 3 weekly injections of PRGF over one long-acting HA at 24 and 48 weeks. PRGF mediates anti-inflammatory effects and the growth factors aid in the repair of the injured cartilage. Raeissadat et al.[38] prepared PRGF by first producing PRP, followed by centrifugation of the upper 2 layers and addition of platelet-activating factor. This led to the release of growth factors by the platelets and subsequently a third spin, which made the platelets and attached fibrin stick to the bottom of the tube. The resultant fluid rich in growth factors was injected intra-articularly in 31 knees. They documented improvement in WOMAC and VAS scores compared to HA (36 knees). They believe that PRGF has the same effects as PRP without its side effects. All studies have reported no severe adverse reactions with only minor complications observed. Raeissadat et al.[38] reported swelling in 1 case and stiffness and heaviness of injection site in 6 cases of PGRF, while Vaquerizo et al.[37] reported pain at the infiltration site in 7 patients.
PRP has been consistently shown to be beneficial in the treatment of OA. However, research needs to be focused now on newer formulations, biomaterials, combinations, and newer modes of deliveries.
Autologous Conditioned Serum
OA is associated with the upregulation of proinflammatory cytokines and matrix metalloproteinases (MMPs), including significant levels of interleukin-1 receptors on synovial fibroblasts and chondrocytes.[39] The interaction of IL-1 with its receptors triggers the inflammatory cascade responsible for OA knee pain and pathogenesis. This IL-1 receptor can be targeted by IL-1 receptor antagonist (IL-1Ra), thereby blocking its signaling activity.[40] Autologous conditioned serum (ACS) is rich in IRAP and hence used in OA Knee.
Meijer et al.[41] were the first to develop ACS branded as “Orthokine”. The patient's whole blood was incubated with glass beads to produce an autologous cell-free serum that is administered intra-articularly twice weekly for 3 weeks. This therapy is available for humans in some European countries and has more widespread use in equine OA, where it improves clinical lameness in horses and has a suggested role of cartilage protection from degradation.[42] Weinberger[43] and Baselga García-Escudero et al.[44] have shown improved functional status with ACS use in animals. Baltzer et al.[8] conducted an RCT with 376 patients (3 groups) and compared ACS with HA and placebo. They reported improved functional outcome scores in the ACS group compared to the baseline and much larger improvement as compared to HA. They noted that the therapeutic effects persist for at least 2 years and noted an overall excellent safety profile. Auw Yang et al.[9] compared ACS with saline controls in 167 patients and observed significant improvement in functionality in both groups with a significant improvement in KOOS score compared with placebo. However, some studies, including Rutgers et al.,[45] have found no difference between placebo and ACS; they postulated that cytokines vanish quickly from the synovial fluid after intra-articular injection. They observed that proinflammatory cytokines were also enhanced along with anti-inflammatory cytokines.
The major issue with ACS is that it is a prolonged process. Baltzer et al.[8] prepared ACS with an incubation period of 24 h and recommended 6 injections (2 mL weekly for 6 weeks). Tassara et al.,[45] in a retrospective series of 28 patients prepared ACS by incubation for 6 h, followed by centrifugation at 5000 rpm for 10 min. They reported a rapid decline in pain with a large improvement in knee range of motion. Woodell May et al.[46] followed a different protocol of ACS preparation, wherein they first prepared PRP from the blood by centrifugation and then incubated the PRP with glass beads. They noted similar desired results with a much shorter incubation period. They concluded that neither time nor temperature significantly increased IL-1Ra production. This was an important finding, as it paved the way for developing ACS in a much shorter time and making it easily available. Barreto et al.[47] centrifuged 60 ml blood for 15 min and then incubated it with medical grade beads for 30 min at ambient room temperature followed by a second spin for 3.5 min, which yielded ACS (Arthrokinex). They reported improvement in pain and functionality at 1 year of follow-up.
King et al.[48] observed that increased WBC concentration correlates with increased concentration of IL-1Ra in their product (nSTRIDE). This high concentration of WBC is achieved using the buffy coat layer after centrifuging blood (LR-PRP). Kon et al.[49] conducted a pilot double-blinded RCT in 46 patients and randomized it into 2 groups: APS group (n = 31) and saline group (n = 15). They reported improvement in functionality, and significant difference between groups was detected in a change in lesion size and central zone osteophytes of the lateral femoral condyle.
ACS has the potential to offer disease-modifying and chondroprotective effects for the management of mild and moderate OA. However, its potency is not validated, and large randomized control trials are required to evaluate its long-term benefits. Nevertheless, its effectiveness in short to medium term with minimal complications is fairly well documented.
Alpha-2 Macroglobulin
A2M is a serum protease inhibitor, inhibiting all classes of endoproteases. These endoproteases include cartilage oligomeric matrix protein-cleaving proteinases (comp), MMP-13 and pro-inflammatory cytokines (IΛ-1 β and tumor necrosis factor-α).[50],[51] It acts as a scavenger molecule by attaching to the proteinases, inducing conformational changes, and thereby scavenging them. They subsequently bind to macrophage receptors and result in clearance of the complex.[52] This resultant chondrogenic and chondroprotective effects have made A2M emerge as a potential therapeutic option for OA treatment. A2M is prepared by passing PRP through various filters, and by sequential filtration, small molecules escape out, and the resultant plasma is rich in A2M, which is a huge molecule.
Clinical trials in humans are underway and yet to be published.[53] However, many studies have reported therapeutic benefits in animal studies. Wang et al.[10] showed rats that underwent anterior cruciate ligament (ACL) transection and received intra-articular A2M had decreased MMP-13 levels and a slower rate of progression of OA. They suggested that supplemental intra-articular A2M provides chondral protection for posttraumatic OA on OA cartilage samples. Cuellar et al.[11] used New Zealand white rabbits and gave intra-articular injections on days 1, 4, and 14 post-ACL transection and showed less joint degeneration and supportive role of A2M in cartilage preservation.
It is postulated that the major beneficial role of A2M may be in the acute flare of OA. This is because the acute flare is associated with the upregulation of proteases, which are inflammatory proteins. A2M inhibits these proteases and neutralizes cartilage degradation and joint destruction. A2M has been referred to as the master inhibitory molecule by Wang et al.[10]
Nevertheless, clinical studies are needed to assess its true potential benefits for knee OA. In this regard, a phase 1 RCT clinical trial is underway in 75 patients to assess the ability of A2M in reducing proinflammatory synovial fluid biomarkers.[53]
Bone Marrow Aspirate Concentrate
Bone marrow aspirate concentrate (BMAC) injections have recently been used for OA knee, owing to the regenerative potential of progenitor cells in marrow.[54] Bone marrow aspiration is percutaneous, safe, and commonly performed from the iliac crest; this is centrifuged to isolate its cellular components in distinct layers. BMAC is rich in mesenchymal stem cells (MSCs), which are capable of differentiation toward cells of a mesodermal lineage. It also has high concentrations of IL-1Ra and IL-1 beta, which are anti-inflammatory growth factors.[55],[56] Although MSCs comprise only 0.001%–0.01% of the cells in BMAC, these have homing abilities, which recruit more cells to the desired site.[57] There is an ongoing discussion to rename MSCs as medicinal signaling cells from its earlier nomenclature of MSCs due to its autocrine and paracrine functions.
One important aspect of BMAC preparation is to obtain a large population of progenitor cells; hence, a good technique of bone marrow aspiration is vital. Hernigou et al.[58] described an improved output with aspiration at multiple locations with a small syringe; however, Oliver et al.[59] found no significant difference in single versus multiple location aspirations. On the other hand, they reported increased procedural pain with multiple site aspiration. Either way, it is important to maintain low aspiration volumes, as the first 2 ml collects the bone marrow-derived cells, and this is diluted by blood volume subsequently.
Few studies have assessed the use of BMAC in OA knees. Kim et al.,[60] in a series of 75 patients, reported increased functional scores (IKDC, KOOS, and SF-36) compared to preoperative scores, although this was not statistically significant. They reported that a higher grade of OA was associated with poorer outcomes. Centeno et al.[61] compared the efficacy of BMAC with adipose tissue derivative (ATD) cells but reported no improvement in efficacy. Shapiro et al.[62] conducted a placebo (saline) controlled pilot study in 25 patients. They reported that BMAC and saline caused similar pain relief and improvement in activity level at 6 months of follow-up. Themistocleous et al.[63] retrospectively analyzed intra-articular BMAC in a series of 121 patients and concluded that it is a safe procedure causing clinical improvement in OA. Anz et al.[64] compared BMAC with PRP in their RCT involving 90 patients and they reported similar improvements in both groups. There was no superiority of BMAC over PRP.
Chahla et al.,[65] in a systemic review, reported good outcomes; however, they highlighted the lack of high-quality studies. Although studies have shown the benefits of BMAC, evidence supporting its superiority to PRP has not been established. Moreover, the morbidity associated with the technique of BMAC aspiration from iliac crest compared to a much simpler PRP preparation technique does not justify BMAC use for OA Knee at present.
BMAC is thus a promising option for OA knee in terms of feasibility and ability to concentrate MSCs for safe use. However, longer follow-up studies and large clinical trials are required to assess the effect of cell count, frequency of treatment, and cell types. It is also important to establish if there is any added supremacy over PRP as BMAC includes cellular components as well.
Adipose-Derived Stromal Cell Therapy
ATDs are considered to be one of the greatest sources of adult stem cells, which have the ability to differentiate into chondrocytes or tenocytes.[66] These are also postulated to contain supportive cells that modulate the microenvironment and aid in regeneration and repair.[67]
Use of ATD is becoming popular as bone marrow harvesting is relatively invasive and associated with donor site morbidity and risk of wound infections.[68] Adipose tissue is abundant and an easily accessible cell source and has characteristics similar to that of bone marrow-derived MSCs.[69] Moreover, MSCs derived from adipose tissue have been suggested to have the highest chondrogenic potential.[70]
These are collected from the lipoaspirate of the abdomen, thigh, or buttock using handheld syringes or machine-generated vacuum pressure and a liposuction cannula. The aspirated adipose tissue undergoes a serial stepwise processing and leads to the extraction of stromal vascular fraction (SVF). The process involved in preparation is more than minimal manipulation and hence faces regulatory issues in some countries. The procedure is done as a single outpatient visit and hence is desirable to the patient. ATD used to treat knee OA includes microfragmented adipose (MFAT) tissue and an SVF. MFAT is generated by mechanically breaking up the adipose tissue by passing it through a size reduction filter.[71] SVF is obtained by centrifugation followed by a-enzymatic digestion (usually collagenase), b-enzymatic digestion, and size reduction filter or c-filter alone.[72] However, it is believed that filter alone lacks the efficiency and yield of enzymatic separation.
There are many studies, which have shown the positive effects of ATDs in OA models in animals. Toghraie et al.[73] induced OA in 20 New Zealand white rabbits and showed that ATD decreased the amount of joint space narrowing, subchondral sclerosis, and osteophyte formation. Mei et al.[74] demonstrated that ASC therapy in a rat model of OA decreased cartilage degeneration grossly and histologically by 8–12 weeks after treatment when compared with a placebo.
Some studies in the literature have reported positive results with the use of ATDs. Spasovski et al.,[75] in their series of 9 patients, reported improvement in all clinical scores and substantial pain relief at 18 months of follow-up. Koh et al.[76] used adipose MSCs in 35 patients and reported good to excellent results in 33 patients. Jo et al.[77] reported that clinical outcomes improved in all 18 patients with the use of intra-articular ATDs. They also studied the relation of three doses of cells with the duration of relief. The clinical scores deteriorated after 1 year in low- (1 × 107) and medium-dose groups (5 × 107), while the scores plateaued and persisted until 2 years in the high-dose group (1 × 108). Adverse effects were minimal in all the above studies, consisting of pain and swelling limited to 24 h.
Culture expansion is not required with ATD use as these already contain a substantially high number of MSCs. Koga et al.[78] used 5 × 107 cells/ml and Wakitani et al.[79] used 1 × 106 cells/ml of culture-expanded MSCs for the treatment of cartilage defects. However, studies using ATDs were able to extract a similar number of cells without culture expansion (Spasovski et al.[75] 0.5–1 × 107; Koh et al.[76] 3.8 × 106 cells/mL).
ATD use has shown early success and acceptable safety profile, but the evidence is low with few studies with small sample sizes available so far. Larger clinical trials are required focusing on aspects of the most effective processing method, cell source, and effective dose. They need further evaluation to arrive at some conclusions.
Gene Therapy
The major challenge with the treatment of OA is that it is an ongoing disease and requires sustained supply and delivery of therapeutic agents (growth factors, IRAP) in order to have sustained benefits; this is where gene therapy could play a major role. Gene therapy aims at the presence of a long-term therapeutic agent to protect and rebuild the damaged articular cartilage. Gene therapy is a promising therapeutic approach for structural modification of OA. All current treatments available are unable to regenerate the entire joint structures completely. However, from the structural aspect, targeting the early stages of OA appears beneficial, as the entire articular cartilage is not yet eroded.
Gene transfer techniques are used to suppress inflammatory factors or to overexpress therapeutic factors (growth factors and transcription factors). Gene delivery is vector based which uses nonviral or viral vectors.[80] Gene transfer is done by anin vivo or ex vivo approach. In vivo, the gene of interest is directly introduced into the patient's own cells within the knee by injection of the viral vector carrying the desired gene. Ex vivo is modification of target cell outside the patient's body and subsequent injection of the same into the knees to express the desired gene. Recombinant adeno-associated virus has emerged as the most promising candidate for both ex vivo andin vivo gene therapy. It is used forin vivo therapy due to its possible long-term clinical benefits.[81]
Ex vivo approach by targeting transforming growth factor beta (TGF-β1) expression is currently the popular gene therapy option for OA knee. It is supported by animal studies as well as Phase 2,[12],[13],[14] Phase 3 clinical trials.[15] In this technique, allogeneic chondrocytes are genetically modified using selective genes (TGF-β1 expression related), which are introduced within them and these allogeneic chondrocytes are available as over-the-counter products for injection.
Noh et al.[82] first described the potential positive effects of chondrocytes expressing TGF-β1 in a preclinical evaluation in animal models with articular damage. They reported new foci of hyaline cartilage matrix with staining characteristics consistent with articular cartilage. Ha et al.[83] conducted the first clinical trial (Phase 1) in patients with 12 end-stage knee OA using a retroviral mediated gene transfer to cause overexpression of TGF β1. They observed minor local reactions, most common being knee effusion but no serious adverse events. This was followed by a multicenter, single-blind Phase 2 trial in which patients were randomized to receive TGF β1 at doses 6 × 106 or 1.8 × 107 cells at a 1:1 ratio.[12] No significant adverse events occurred and both groups showed improvement in functional scores (IKDC and WOMAC) and VAS score. Lee et al.[13] conducted a placebo RCT and reported significantly improved IKDC and VAS score in the gene therapy group as compared to placebo. Cherian et al.[14] conducted a Phase 2 randomized study of genetically engineered allogeneic human chondrocytes expressing TGF β1 in patients with grade 3 OA in 102 patients and reported a more positive response in IKDC evaluation, VAS, and less likely use of analgesics.
Kim et al.[15] conducted a Phase 3 clinical trial using retrovirally transduced chondrocytes to overexpress TGF β1. They reported significant improvement in IKDC and VAS scores and trends toward thicker cartilage on MRI. In fact, South Korea approved the world's first gene therapy (Invossa), which encodes transforming growth factor-β1 in 2018.[84],[85]
Research exploringin vivo pathways of gene therapy is usually directed toward IL-1 pathway and is still in its infancy stages with fewin vivo studies available. Nixon et al.[86] conducted a study on mouse and horse models expressing the IL-1Ra gene. They noted improvement in the cartilage volume and surface in mice and improved lameness parameters in horses. Currently, clinical trials are underway for IL1Ra based gene therapy.[87]
Gene therapy strategies, thus, enable targeted gene delivery and bear promise for the future to alleviate symptoms in early OA as it has the potential for sustained delivery of the drug. It may prove to be the option that may reverse or at least halt the disease process by structural disease modifications. Research into region-specific editing of genes related to OA is ongoing and may allow controlled therapeutic gene expression for OA treatment.
Conclusion
The success and safety of PRP use in OA Knee have been encouraging and have prompted research into newer Orthobiologics substitutes for intra-articular use. Orthobiologics are encouraging as they are believed to be disease-modifying therapy strategies. Trends are focused on using specific growth factor extracts, cellular therapy (MSCs from BMAC and ATD). Gene therapy is in Phase 3 clinical trials with early promising results. The debate is ongoing regarding the benefit of these orthobiologics and further studies are needed to clarify their role.
Ethical approval
The authors confirm that this review had been prepared in accordance to COPE roles and regulation. Given the nature of the review, IRB review was not required.
Financial support and sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
There are no conflicts of interest.
Authors contribution
SP analyzed data and reviewed the initial draft and provided logistic support. KJ wrote initial and final draft. MD Conceived and designed the study and provided research material. All authors have critically reviewed and approved the final draft and are responsible for the manuscript's content and similarity index.
| 1. | Lana JF, de Castro RB, Rodrigues BL, Caliari C. Orthobiologic treatment for knee osteoarthritis: A cost effectiveness choice. Biomed J 2019;1:7. [Google Scholar] |
| 2. | Huebner K, Frank RM, Getgood A. Ortho-biologics for osteoarthritis. Clin Sports Med 2019;38:123-41. [Google Scholar] |
| 3. | AAOS. 2010. Available from: https://orthoinfo.aaos.org/en/treatment/helping-fractures-heal-orthobiologics. [Last accessed on 2020 May 17]. [Google Scholar] |
| 4. | Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: A prospective, double-blind, randomized trial. Am J Sports Med 2013;41:356-64. [Google Scholar] |
| 5. | Laudy AB, Bakker EW, Rekers M, Moen MH. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: A systematic review and meta-analysis. Br J Sports Med 2015;49:657-72. [Google Scholar] |
| 6. | Dai WL, Zhou AG, Zhang H, Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: A meta-analysis of randomized controlled trials. Arthroscopy 2017;33:659-700. [Google Scholar] |
| 7. | Chang KV, Hung CY, Aliwarga F, Wang TG, Han DS, Chen WS. Comparative effectiveness of platelet-rich plasma injections for treating knee joint cartilage degenerative pathology: A systematic review and meta-analysis. Arch Phys Med Rehabil 2014;95:562-75. [Google Scholar] |
| 8. | Baltzer AW, Moser C, Jansen SA, Krauspe R. Autologous conditioned serum (orthokine) is an effective treatment for knee osteoarthritis. Osteoarthritis Cartilage 2009;17:152-60. [Google Scholar] |
| 9. | Auw Yang KG, Raijmakers NJ, van Arkel ER, Caron JJ, Rijk PC, Willems WJ, et al. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthritis Cartilage 2008;16:498-505. [Google Scholar] |
| 10. | Wang S, Wei X, Zhou J, Zhang J, Li K, Chen Q, et al. Identification of α2-macroglobulin as a master inhibitor of cartilage-degrading factors that attenuates the progression of posttraumatic osteoarthritis. Arthritis Rheumatol2014;661843-53. [Google Scholar] |
| 11. | Cuellar JM, Browning SR, Cuellar VG, Golish SR, Hanna L, Scuderi G. Poster 10 is there a chondroprotective effect of autologous platelet integrated concentrate (APIC) on an osteoarthritis (OA) rabbit model? A pilot study. PMR 2012;4(10):S192. [Google Scholar] |
| 12. | Ha CW, Cho JJ, Elmallah RK, Cherian JJ, Kim TW, Lee MC, et al. A multicenter, single-blind, phase IIa clinical trial to evaluate the efficacy and safety of a cell-mediated gene therapy in degenerative knee arthritis patients. Hum Gene Ther Clin Dev 2015;26:125-30. [Google Scholar] |
| 13. | Lee MC, Ha CW, Elmallah RK, Cherian JJ, Cho JJ, Kim TW et al. A placebo-controlled randomised trial to assess the effect of TGF-ß1-expressing chondrocytes in patients with arthritis of the knee. Bone Joint J 2015;97:924-32. [Google Scholar] |
| 14. | Cherian JJ, Parvizi J, Bramlet D, Lee KH, Romness DW, Mont MA. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-β1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthritis Cartilage 2015;23:2109-18. [Google Scholar] |
| 15. | Kim MK, Ha CW, In Y, Cho SD, Choi ES, Ha JK, et al. A multicenter, double-blind, phase III clinical trial to evaluate the efficacy and safety of a cell and gene therapy in knee osteoarthritis patients. Hum Gene Ther Clin Dev 2018;29:48-59. [Google Scholar] |
| 16. | Sundman EA, Cole BJ, Karas V, Della Valle C, Tetreault MW, Mohammed HO, et al. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med 2014;42:35-41. [Google Scholar] |
| 17. | Sánchez M, Anitua E, Azofra J, Aguirre JJ, Andia I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: A retrospective cohort study. Clin Exp Rheumatol 2008;26:910-3. [Google Scholar] |
| 18. | Spaková T, Rosocha J, Lacko M, Harvanová D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil 2012;91:411-7. [Google Scholar] |
| 19. | Filardo G, Kon E, Pereira Ruiz MT, Vaccaro F, Guitaldi R, Di Martino A, et al. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: Single-versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc 2012;20:2082-91. [Google Scholar] |
| 20. | Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med 2016;44:792-800. [Google Scholar] |
| 21. | Arnoczky SP, Delos D, Rodeo SA. What is platelet-rich plasma? Oper Tech Sports Med 2011;19:142-8. [Google Scholar] |
| 22. | Kavadar G, Demircioglu DT, Celik MY, Emre TY. Effectiveness of platelet-rich plasma in the treatment of moderate knee osteoarthritis: A randomized prospective study. J Phys Ther Sci 2015;27:3863-7. [Google Scholar] |
| 23. | Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: A randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc 2017;25:958-65. [Google Scholar] |
| 24. | Mazzocca AD, McCarthy MB, Chowaniec DM, Cote MP, Romeo AA, Bradley JP, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am 2012;94:308-16. [Google Scholar] |
| 25. | Saturveithan C, Premganesh G, Fakhrizzaki S, Mahathir M, Karuna K, Rauf K, et al. Intra-articular hyaluronic acid (HA) and platelet rich plasma (PRP) injection versus Hyaluronic acid (HA) injection alone in patients with Grade III and IV knee osteoarthritis (OA): A retrospective study on functional outcome. Malays Orthop J 2016;10:35-40. [Google Scholar] |
| 26. | Russo F, D'Este M, Vadalà G, Cattani C, Papalia R, Alini M, et al. Platelet Rich Plasma and Hyaluronic Acid Blend for the Treatment of Osteoarthritis: Rheological and Biological Evaluation. PLoS One 2016;11:e0157048. [Google Scholar] |
| 27. | Patel S, Dhillon MS, Bansal T. Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee-Letter to the Editor & author response. J Stem Cells Regen Med 2017;13:80-3. [Google Scholar] |
| 28. | Saito M, Takahashi KA, Arai E, Inoue A, Sakao K, Tonomura H, et al. Intra-articular administration of platelet-rich plasma with biodegradable gelatin hydrogel microspheres prevents osteoarthritis progression in the rabbit knee. Clin Exp Rheumatol 2009;27:201. [Google Scholar] |
| 29. | Dwivedi G, Chevrier A, Hoemann CD, Buschmann MD. Injectable freeze-dried chitosan-platelet-rich-plasma implants improve marrow-stimulated cartilage repair in a chronic-defect rabbit model. J Tissue Eng Regen Med 2019;13:599-611. [Google Scholar] |
| 30. | Paterson KL, Nicholls M, Bennell KL, Bates D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: A double-blind, randomized controlled pilot study. BMC Musculoskelet Disord 2016;17:67. [Google Scholar] |
| 31. | Sánchez M, Fiz N, Guadilla J, Padilla S, Anitua E, Sánchez P, et al. Intraosseous infiltration of platelet-rich plasma for severe knee osteoarthritis. Arthrosc Tech 2014;3:e713-7. [Google Scholar] |
| 32. | Sanchez M, Delgado D, Sanchez P, Muinos-Lopez E, Paiva B, Granero-Molto F, et al. Combination of intra-articular and intraosseous injections of platelet rich plasma for severe knee osteoarthritis: A pilot study. BioMed Res Int 2016;2016:4868613. [Google Scholar] |
| 33. | Sánchez M, Delgado D, Pompei O, Pérez JC, Sánchez P, Garate A, et al. Treating severe knee osteoarthritis with combination of intra-osseous and intra-articular infiltrations of platelet-rich plasma: An observational study. Cartilage 2019;10:245-53. [Google Scholar] |
| 34. | Su K, Bai Y, Wang J, Zhang H, Liu H, Ma S. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin Rheumatol 2018;37:1341-50. [Google Scholar] |
| 35. | Bottegoni C, Dei Giudici L, Salvemini S, Chiurazzi E, Bencivenga R, Gigante A. Homologous platelet-rich plasma for the treatment of knee osteoarthritis in selected elderly patients: An open-label, uncontrolled, pilot study. Ther Adv Musculoskelet Dis 2016;8:35-41. [Google Scholar] |
| 36. | Anitua E, Sanchez M, De la Fuente M, Zalduendo MM, Orive G. Plasma rich in growth factors (PRGF-Endoret) stimulates tendon and synovial fibroblasts migration and improves the biological properties of hyaluronic acid. Knee Surg Sports Traumatol Arthrosc 2012;20:1657-65. [Google Scholar] |
| 37. | Vaquerizo V, Plasencia MÁ, Arribas I, Seijas R, Padilla S, Orive G, et al. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus Durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: A randomized controlled trial. Arthroscopy 2013;29:1635-43. [Google Scholar] |
| 38. | Raeissadat SA, Rayegani SM, Ahangar AG, Abadi PH, Mojgani P, Ahangar OG. Efficacy of intra-articular injection of a newly developed plasma rich in growth factor (PRGF) versus hyaluronic acid on pain and function of patients with knee osteoarthritis: A single-blinded randomized clinical trial. Clin Med Insights Arthritis Musculoskelet Disord 2017;10:1179544117733452. [Google Scholar] |
| 39. | Wassilew GI, Lehnigk U, Duda GN, Taylor WR, Matziolis G, Dynybil C. The expression of proinflammatory cytokines and matrix metalloproteinases in the synovial membranes of patients with osteoarthritis compared with traumatic knee disorders. Arthroscopy 2010;26:1096-104. [Google Scholar] |
| 40. | Dinarello CA, Thompson RC. Blocking IL-1: Interleukin 1 receptor antagonistin vivo and in vitro. Immunol Today 1991;12:404-10. [Google Scholar] |
| 41. | Meijer H, Reinecke J, Becker C, Tholen G, Wehling P. The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm Res 2003;52:404-7. [Google Scholar] |
| 42. | Bertone AL, Ishihara A, Zekas LJ, Wellman ML, Lewis KB, Schwarze RA, et al. Evaluation of a single intra-articular injection of autologous protein solution for treatment of osteoarthritis in horses. Am J Vet Res 2014;75:141-51. [Google Scholar] |
| 43. | Weinberger T. Clinical experience with ACS/Orthokine/IRAP in horses. Equine Sports Med 2008;3:1-5. [Google Scholar] |
| 44. | Baselga García-Escudero J, Miguel Hernández Trillos P. Treatment of osteoarthritis of the knee with a combination of autologous conditioned serum and physiotherapy: A two-year observational study. PLoS One 2015;10:e0145551. [Google Scholar] |
| 45. | Tassara M, De Ponti A, Barzizza L, Zambelli M, Parisi C, Milani R, et al. Autologous conditioned serum (ACS) for intra-articular treatment in osteoarthritis: Retrospective report of 28 cases. Transfus Apher Sci 2018;57:573-7. [Google Scholar] |
| 46. | Woodell-May J. “Effect of Incubation Time on Production of IL-1ra and sTNF-RI from Platelet-Rich Plasma” Paper No. 200, 55th Annual Meeting of the Orthopaedic Research Society; February, 2009. p. 1. [Google Scholar] |
| 47. | Barreto A, Braun TR. A method to induce interleukin-1 receptor antagonist Protein from autologous whole blood. Cytokine 2016;81:137-41. [Google Scholar] |
| 48. | King W, van der Weegen W, Van Drumpt R, Soons H, Toler K, Woodell-May J. White blood cell concentration correlates with increased concentrations of IL-1ra and improvement in WOMAC pain scores in an open-label safety study of autologous protein solution. J Exp Orthop 2016;3:9. [Google Scholar] |
| 49. | Kon E, Engebretsen L, Verdonk P, Nehrer S, Filardo G. Clinical outcomes of knee osteoarthritis treated with an autologous protein solution injection: A 1-year pilot double-blinded randomized controlled trial. Am J Sports Med 2018;46:171-80. [Google Scholar] |
| 50. | Wan R, Hu J, Zhou Q, Wang J, Liu P, Wei Y. Application of co-expressed genes to articular cartilage: New hope for the treatment of osteoarthritis (review). Mol Med Rep 2012;6:16-8. [Google Scholar] |
| 51. | Luan Y, Kong L, Howell DR, Ilalov K, Fajardo M, Bai XH, et al. Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matrix protein by alpha-2-macroglobulin. Osteoarthritis Cartilage 2008;16:1413-20. [Google Scholar] |
| 52. | Cuellar JM, Cuellar VG, Scuderi GJ. α2-microglobulin autologous protease inhibition technology. Phys Med Rehabil Clin N Am 2016;27:909-18. [Google Scholar] |
| 53. | ClinicalTrials. Gov. Bethesda (MD): National Library of Medicine (US). February 29, 2000. Identifier NCT03656575, Reduction of Pro-Inflammatory Synovial Fluid Biomarkers in Osteoarthritis of the Knee with Alpha-2 Macroglobulin; September 4, 2018. Available from: https://clinicaltrials. gov/ct2/show/record/NCT03656575. [Last cited on 2020 May 31]. [Google Scholar] |
| 54. | Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076-84. [Google Scholar] |
| 55. | Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am 2010;92:1927-37. [Google Scholar] |
| 56. | Oliver KS, Bayes M, Crane D, Pathikonda C. Clinical outcome of bone marrow concentrate in knee osteoarthritis. J Prolother. 2015;7:e937-46. [Google Scholar] |
| 57. | Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel mono-clonal antibody, STRO-1. Blood 1991;78:55-62. [Google Scholar] |
| 58. | Hernigou P, Homma Y, Flouzat Lachaniette CH, Poignard A, Allain J, Chevallier N, et al. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop 2013;37:2279-87. [Google Scholar] |
| 59. | Oliver K, Awan T, Bayes M. Single-versus multiple-site harvesting techniques for bone marrow concentrate: Evaluation of aspirate quality and pain. Orthop J Sports Med 2017;5:2325967117724398. [Google Scholar] |
| 60. | Kim JD, Lee GW, Jung GH, Kim CK, Kim T, Park JH, et al. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur J Orthop Surg Traumatol 2014;24:1505-11. [Google Scholar] |
| 61. | Centeno C, Pitts J, Al-Sayegh H, Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed Res Int 2014;2014:370621. [Google Scholar] |
| 62. | Shapiro SA, Kazmerchak SE, Heckman MG, Zubair AC, O'Connor MI. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med 2017;45:82-90. [Google Scholar] |
| 63. | Themistocleous GS, Chloros GD, Kyrantzoulis IM, Georgokostas IA, Themistocleous MS, Papagelopoulos PJ, et al. Effectiveness of a single intra-articular bone marrow aspirate concentrate (BMAC) injection in patients with grade 3 and 4 knee osteoarthritis. Heliyon 2018;4:e00871. [Google Scholar] |
| 64. | Anz AW, Hubbard R, Rendos NK, Everts PA, Andrews JR, Hackel JG. Bone marrow aspirate concentrate is equivalent to platelet-rich plasma for the treatment of knee osteoarthritis at 1 year: A prospective, randomized trial. Orthop J Sports Med 2020;8:2325967119900958. [Google Scholar] |
| 65. | Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: A systematic review of outcomes. Orthop J Sports Med 2016;4:2325967115625481. [Google Scholar] |
| 66. | Alderman DD, Alexander RW. Advances in regenerative medicine: High-density platelet-rich plasma and stem cell prolotherapy for musculoskeletal pain. Pract Pain Manag 2011;11:49-63, 90. [Google Scholar] |
| 67. | Oberbauer E, Steffenhagen C, Wurzer C, Gabriel C, Redl H, Wolbank S. Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: Current state of the art. Cell Regen 2015;4:7. [Google Scholar] |
| 68. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143-7. [Google Scholar] |
| 69. | Guilak F, Awad HA, Fermor B, Leddy HA, Gimble JM. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology 2004;41:389-99. [Google Scholar] |
| 70. | Estes BT, Diekman BO, Gimble JM, Guilak F. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc 2010;5:1294-311. [Google Scholar] |
| 71. | Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 2013;22:2063-77. [Google Scholar] |
| 72. | Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res Ther 2017;8:145. [Google Scholar] |
| 73. | Toghraie FS, Chenari N, Gholipour MA, Faghih Z, Torabinejad S, Dehghani S, et al. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit. Knee 2011;18:71-5. [Google Scholar] |
| 74. | Mei L, Shen B, Ling P, Liu S, Xue J, Liu F, et al. Culture-expanded allogenic adipose tissue-derived stem cells attenuate cartilage degeneration in an experimental rat osteoarthritis model. PLoS One 2017;12:e0176107. [Google Scholar] |
| 75. | Spasovski D, Spasovski V, Baščarević Z, Stojiljković M, Vreća M, Anđelković M, et al. Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. J Gene Med 2018;20:e3002. [Google Scholar] |
| 76. | Koh YG, Choi YJ, Kwon OR, Kim YS. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med 2014;42:1628-37. [Google Scholar] |
| 77. | Jo CH, Chai JW, Jeong EC, Oh S, Shin JS, Shim H, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A 2-year follow-up study. Am J Sports Med 2017;45:2774-83. [Google Scholar] |
| 78. | Koga H, Shimaya M, Muneta T, Nimura A, Morito T, Hayashi M, et al. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res Ther 2008;10:R84. [Google Scholar] |
| 79. | Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage 2002;10:199-206. [Google Scholar] |
| 80. | Evans CH, Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat Rev Rheumatol 2015;11:234-42. [Google Scholar] |
| 81. | Rey-Rico A, Cucchiarini M. Smart and controllable rAAV gene delivery carriers in progenitor cells for human musculoskeletal regenerative medicine with a focus on the articular cartilage. Curr Gene Ther 2017;17:127-38. [Google Scholar] |
| 82. | Noh MJ, Copeland RO, Yi Y, Choi KB, Meschter C, Hwang S, et al. Pre-clinical studies of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 (TG-C). Cytotherapy 2010;12:384-93. [Google Scholar] |
| 83. | Ha CW, Noh MJ, Choi KB, Lee KH. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy 2012;14:247-56. [Google Scholar] |
| 84. | Ji-Young S. Korea OKsFirst Cell Gene Therapy 'Invossa.' The Korea Herald. July 12, 2017. [Google Scholar] |
| 85. | Evans CH, Ghivizzani SC, Robbins PD. Arthritis gene therapy approved in Korea. J Am Acad Orthop Surg 2018;26:e36-8. [Google Scholar] |
| 86. | Nixon AJ, Grol MW, Lang HM, Ruan MZC, Stone A, Begum L, et al. Disease-modifying osteoarthritis treatment with interleukin-1 Receptor antagonist gene therapy in small and large animal models. Arthritis Rheumatol 2018;70:1757-68. [Google Scholar] |
| 87. | Available from: https://www.mayo.edu/research/clinical-trials/cls-20258269_ga=2.176734306.612216708.1573153930-2113802835.1573153930. [Last accessed on 2020 May 05]. [Google Scholar] |
Fulltext Views
4,262
PDF downloads
2,275





