Translate this page into:
The infraclavicular approach for neurectomy of the spastic shoulder
2 Department of Plastic and Reconstructive Surgery, Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK
3 Upper Limb Spasticity Service, Department of Hand Surgery, Royal Orthopaedic Hospital, Birmingham, UK
Corresponding Author:
Dominic M Power
Birmingham Hand Centre, Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust, West Midlands Brachial Plexus and Peripheral Nerve Injury Service, 6th Floor, Nuffield House, Mindelsohn Way, Edgbaston, Birmingham B15 2WB
UK
dominic.power@uhb.nhs.uk
| How to cite this article: Power DM, Nassimizadeh M, Mikalef P. The infraclavicular approach for neurectomy of the spastic shoulder. J Musculoskelet Surg Res 2019;3:161-165 |
Abstract
Objectives: Total or partial motor neurectomy may be used in the management of recalcitrant spasticity. A shoulder posture with adduction and internal rotation, impair function, and left untreated may result in established contractures. The aims of this anatomical study and clinical case series were to define a safe surgical approach to the infraclavicular plexus for selected motor neurectomy that avoids the axillary skin and can be readily completed in the presence of early axillary contractures. Methods: Using a cadaveric model, we adopted the pectoralis major muscle-splitting approach to the brachial plexus to afford access to the infraclavicular motor branches to the shoulder. The pectoral nerve origins can be identified and traced to their respective cords. Following pectoralis minor tenotomy, the interval between the lateral cord and the axillary artery is developed to expose the posterior cord. The subscapular and thoracodorsal nerves are identified using nerve stimulation. Results: The procedure has been used in the four non-functional limbs with shoulder adduction and internal rotation deformity with early axillary contractures resistant to physiotherapy and splinting. A mean of 6.25 nerves were sectioned in each case. All patients or their caregivers reported improvements in shoulder posture and pain with examination, demonstrating a mean increase passive in brachiothoracic angle of 45° (range: 30°–60°) facilitating washing and dressing. The improvements have been maintained at 12-month follow-up. Conclusion: Management of the painful adducted and internally rotated spastic shoulder is challenging. A mini-incision pectoralis major splitting incision provides access to the key motor nerves to the shoulder for neurectomy. Comparative studies evaluating neurectomy and tenotomy at the shoulder are required to demonstrate efficacy.Introduction
Spasticity may be associated with stroke, cerebral palsy, or traumatic brain injury. The pathophysiological processes causing spasticity are not fully understood; however, there is muscle imbalance, abnormal joint posture with contracture risk, with movement, and functional impairment.
The post-stroke upper limb typically has a postural deformity encompassing shoulder adduction and internal rotation, elbow flexion, forearm pronation, wrist and digit flexion, and thumb flexion and adduction. The poor shoulder position negatively impacts on the upper limb use in otherwise potentially functional participants and may cause pain and axillary hygiene concerns for those with a non-functional spastic upper limb. The early phase of post-stroke management includes constraint-induced therapy, passive stretches, and preventative splinting techniques. Pharmacological denervation with botulinum toxin may delay or prevent the onset of spasticity, and in established spasticity, the tone reduction can assist rehabilitation but must be balanced against the loss of useful tone and strength in functional patients.[1] Treatment is individualized and depends on the degree of physical and cognitive impairment, the functional potential, and the general medical condition of the patient.
In the non-functional limb without volitional control, hygiene and difficulties in dressing are challenges facing both patients and their care provider. In these patients, surgical intervention may prevent contracture formation or may be used to release established contractures and correct postural deformity. Procedures aimed at reducing the deforming forces across joints include muscle and tendon lengthening, tendon transfers, and surgical neurectomies.[2]
In the non-functional shoulder, the hypertonicity of pectoralis major, latissimus dorsi, teres major, and subscapularis muscles results in shoulder adduction and internal rotation. The tendons of these muscles can be released reducing pain, improving passive range of motion, axillary hygiene, and ease of washing and dressing.[3],[4] Additional benefits of the upper limb tone reduction include improved lower limb function, cadence, and reduced energy expenditure of walking. Complications of tenotomy include poor wound healing, joint subluxation, and recurrence of contractures.
Motor neurectomies [Figure - 1] in the infraclavicular brachial plexus may achieve the same result in patients with high tone, but no established contractures or can be used to facilitate passive stretching and splinting in mild contractures. In the non-functional upper limb, total motor neurectomy of the muscle nerve branches of the affected muscles may be used to reduce tone, reduce pain, improve hygiene, and ease of dressing.[5] The surgery is performed remote to the site of the contracted muscles through normal skin. Sectioning pure motor branches allow control of tone without risking sensory disturbance or neuropathic pain that may follow neurectomy of a mixed nerve trunk. The technique of neurectomy is not new.[6] There are advantages over chemical denervation due to consistency in denervation and prolonged duration. Botulinum toxin is associated with fluctuations in tone, strength, and efficacy that are frustrating for the functional patient.[7]
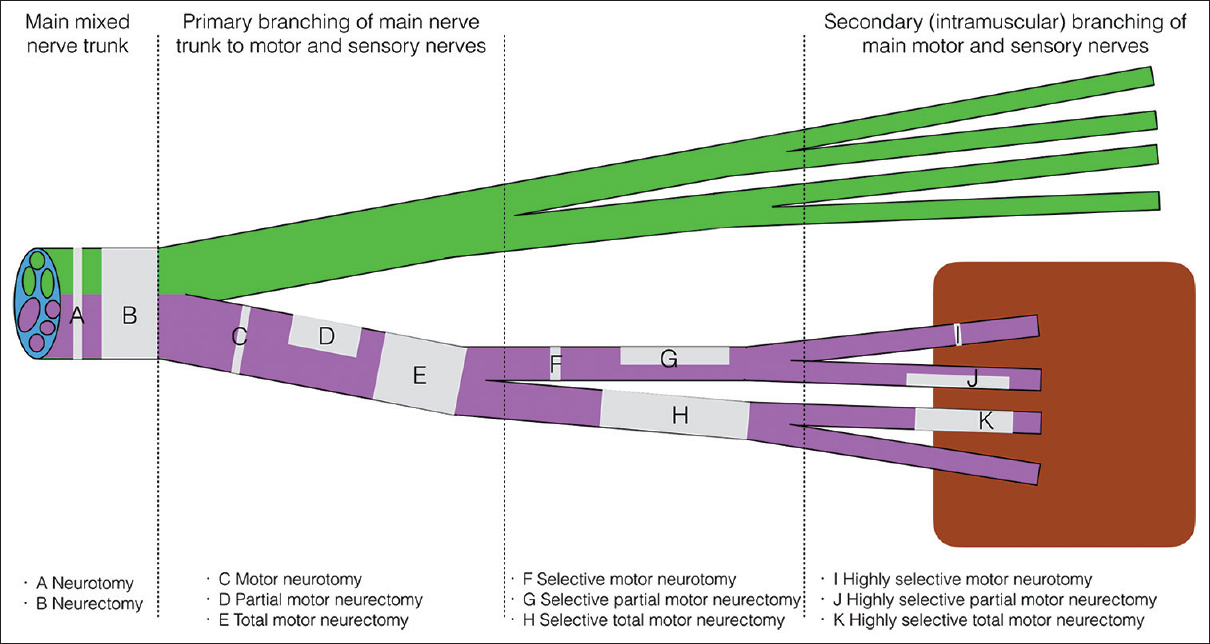 |
| Figure 1: Lexicon for neurectomy |
The evidence for surgical neurectomy in the management algorithm for the upper limb spasticity is emerging;[8] however, for surgeons unfamiliar with the motor branching anatomy of the upper limb nerves, there is a significant learning curve. Peripheral nerve specialists are well positioned to support spasticity surgeons in the incorporation of neurectomy and highly selective neurectomy into management algorithms and to extend the technique into the brachial plexus where the results of tenotomy are inconsistent.
Materials and Methods
Using four fresh cadaveric upper limbs, the feasibility of the pectoralis major approach for access to the required shoulder motor branches was assessed. In addition, both the upper limbs of a preserved anatomical specimen cadaver were dissected to demonstrate the innervation of the key muscles and further define the approach to these nerves. The surgical approach is familiar to brachial plexus surgeons and involves identification of the relatively avascular interval between the clavicular and sternocostal heads of the pectoralis major [Figure - 2]. The skin incision is positioned in a transverse orientation 4 cm below the clavicle along the middle 3/5th of the clavicle. The interval between the muscle heads is developed, and a self-retaining retractor placed to expose the pectoralis minor inserting at the coracoid.
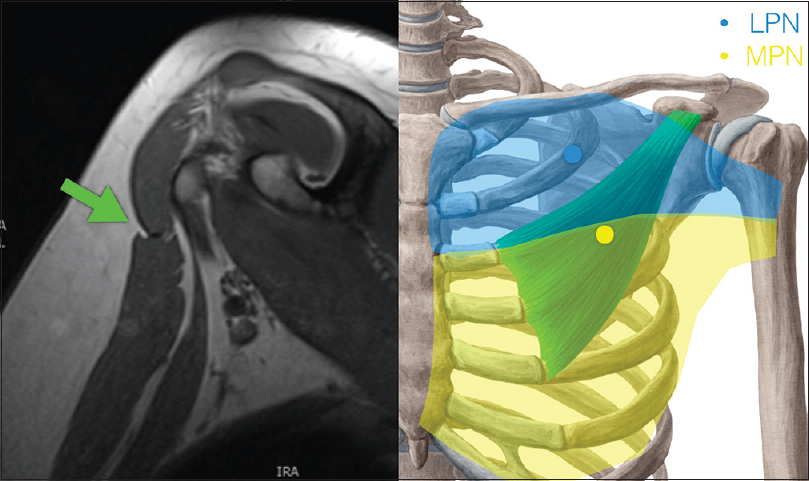 |
| Figure 2: The transpectoral approach to the infraclavicular plexus |
The upper head of pectoralis major is innervated through lateral pectoral nerve branches that pass through the clavipectoral fascia above the pectoralis minor accompanied by the thoracoacromial vessel and the cephalic vein. These branches are described as arising from the lateral cord of the brachial plexus from which they are named. A silicone elastic loop is placed around the nerve branches to identify them for later neurectomy. The interval between the sternocostal head of pectoralis major and the pectoralis minor is developed looking for the medial pectoral nerve branches which typically pass from the medial cord of the brachial plexus, through the pectoralis minor which they innervate to the pectoralis major lower fibers. Accessory branches of the medial pectoral nerves may pass directly to the lower pectoralis major muscle lateral to the pectoralis minor and should be identified. The nerve branches are tagged with surgical loops. Aszmann redefined the anatomy of the pectoral nerves in a cadaveric study in 2000, defining the superior pectoral nerve taking origin from the anterior division of the upper trunk and supplying the lateral clavicular head of the pectoralis muscle. The middle pectoral nerve was identified as taking origin from the anterior division of the middle trunk and dividing into two branches, the superficial branch supplying the medial part of the clavicular head originating from the sternum, and the deep branch joining with the inferior pectoral nerve from the anterior division of the lower trunk to form a network of branches that supply pectoralis minor. In two-third of the cases, the lower pectoralis major was supplied by the pectoral branches piercing pectoralis minor, and in one-third, the supply was through direct branches lateral to the pectoralis minor.[9] The relevance of this work is that there may be a separate branch to the upper medial pectoralis major that passes through the clavipectoral fascia independently of the traditionally named lateral pectoral nerve, and all branches should be traced deep to the cord or trunk of origin if a total neurectomy is planned.
Tenotomy of the pectoralis minor and distal reflection on its muscle origin exposes the infraclavicular brachial plexus. The lateral cord is tagged and followed laterally to identify the origin of the lateral head of the median nerve, the musculocutaneous nerve origin, and the branch to the coracobrachialis. In the typical anatomy, the interval between the lateral cord and the upper axillary artery may be developed to expose the posterior cord. This is a relatively avascular plane and avoids the veins sited medial and inferior to the artery. In cases where the takeoff of the lateral head of the median nerve is proximally located, the lateral cord must be mobilized away from the coracoid and clavicle allowing access to the posterior cord through this alternate plane. The nerve to coracobrachialis will need mobilizing, and the musculocutaneous nerve will need neurolysis and decompression where it passes through the conjoint tendon 4 cm inferior to the coracoid. This alternate plane may cause traction on the lateral pectoral nerve which should therefore be mobilized to facilitate retraction. The axillary nerve takeoff from the posterior cord is the first branch identified in this approach and is tagged and traced proximally to identify the posterior cord.
The tags on the medial pectoral nerve should be reapplied deep to pectoralis minor on the common origin from the medial cord or around each identified separate trunk from the medial cord. The lateral cord origin of the lateral pectoral nerves can be tagged in the same way if neurolysis has not already been completed.
The posterior cord is tagged and retracted superiorly. The axillary artery is tagged with a double surgical loop in case vascular control is required. This may be used to retract the vessel inferiorly to expose the nerve branches arising from the inferior surface of the posterior cord proximal to the axillary artery. The upper subscapular nerve, the thoracodorsal nerve, and the lower subscapular nerve should be identified and tagged in preparation for neurectomy. In clinical practice, the use of a nerve stimulator can confirm the anatomy without needing extensive dissection of each branch to muscle [Figure - 3]. The anatomy of the nerve branches from the posterior cord is variable with the typical arrangement revealing the thoracodorsal nerve lying between the subscapular nerves; however, a common variation is for the thoracodorsal nerve to arise from the axillary nerve, and this anatomy must be confirmed with great care to avoid inadvertent injury to the axillary nerve when undertaking the neurectomy.[10] The teres major innervation is typically from the lower subscapular nerve; however, in 13% of participants, the innervation is from the thoracodorsal nerve.[11]
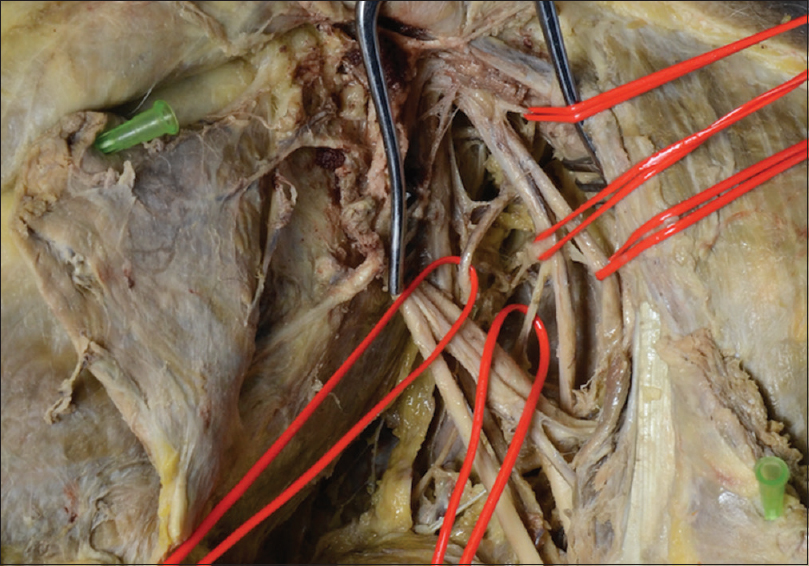 |
| Figure 3: Left infraclavicular plexus exposure: the lateral cord is retracted superiorly and the lower two silicone loops are around a double thoracodorsal nerve with a lower subscapular nerve pedicle between giving origin to the nerve to teres major |
Total or partial motor neurectomy can be performed of the identified lateral pectoral nerve, medial pectoral nerve, upper subscapular nerve, lower subscapular nerve, and thoracodorsal nerve as indicated for management of the shoulder spasticity.
Following the feasibility study, the procedure was performed in four shoulders with spastic deformity as a part of multilevel surgery. Surgery was performed under general anesthesia without neuromuscular blockade so that an intraoperative nerve stimulator could be used to confirm the nerve anatomy.
Results
Four procedures have been performed in four non-functional upper limbs. The cause of the spasticity was post-stroke in two cases and one case each of traumatic brain injury and cerebral palsy. All cases were for total selective motor neurectomies of the pectoral muscles, teres major, subscapularis, and latissimus dorsi. A total of 24 total motor neurectomies (mean: 6.25) were performed as demonstrated in [Table - 1]. A 10-mm section of each nerve was excised for the total neurectomy.

All patients demonstrated significant improvement in the shoulder posture with a mean increase in passive brachiothoracic angle of 45° (range: 30°–60°) facilitating washing and dressing. Three patients reported a reduction in shoulder pain, and for the patient with traumatic brain injury, the primary care provider reported an apparent reduction in discomfort during washing and dressing. There were no complications of the neurectomy procedures. The improvements have been maintained at 12-month follow-up without the need for secondary tenotomy surgery at the shoulder.
Discussion
The upper limb spasticity may result in adduction and internal rotation of the shoulder, impairing upper limb function, and restricting the activities of daily living. Management strategies include mobilization to maintain passive joint range of motion, splints, and chemodenervation using botulinum toxin. Failed nonoperative management results in contracture development, shoulder pain, and poor axillary hygiene. Later, referral for a surgical opinion limits intervention to tenotomies, which must be performed around the shoulder and in proximity to the poor quality skin of the axilla. Although useful, tenotomies may not fully address joint contractures, may result in residual deformity or recurrent contractures. There is a developing trend to earlier intervention with botulinum toxin after stroke to prevent the onset of spasticity or reduce its severity. There are demonstrable benefits from this approach at the elbow; however, efficacy at the shoulder has not yet been demonstrated.[1] Following stroke, the neurological recovery is complete at 6 months, and there is evidence to support the assessment by a spasticity surgeon as part of the multiprofessional team. Intervention at this early stage with neurectomy may reduce the risk of contracture development and provide a more consistent and permanent solution to the spasticity that can be achieved through repeated chemodenervation, which typically results in periods of fluctuant tone and weakness. The absolute indications for neurectomy, hyperselective motor neurectomy (incomplete denervation of a muscle by sectioning some of the motor branches), joint releases, tenotomy, and fractional lengthening are yet to be defined and treatment algorithms are likely to include all of these techniques for multilevel surgery. The evidence for neurectomy for the elbow flexors is more established,[12] and comparative studies are needed to demonstrate a superior outcome and improved patient acceptability of neurectomy over tenotomy and tendon lengthening alone. The trend toward earlier surgical assessment will facilitate a neurectomy approach to shoulder spasticity and prevention of contracture formation.[13] The surgical approach presented here is a technically feasible method of reducing tone in spastic muscles around the shoulder, and efficacy has been demonstrated in four cases without complications.
Conclusion
Total or partial motor neurectomy is a useful strategy in the algorithm for management of the spastic upper limb. The technique may be applied to the shoulder with adduction and internal rotation posture or contracture as an alternative strategy to tendon releases and may be accomplished through a small infraclavicular pectoralis major muscle splitting approach. The technique improves shoulder posture, reduces adduction tone, reduces pain, and facilitates activities of daily living. Comparative studies of neurectomy and tenotomy are required to demonstrate efficacy.
Presentation
This work was presented in part as a poster at the Eurohand Congress, Federation of European Societies for Surgery of the Hand, Copenhagen 2018. The poster was shortlisted for the best poster prize and an invited oral presentation was made at the same meeting.
Ethical consideration
The anatomical dissections were completed in line with internal board review and the clinical case series was performed in accordance with local governance guidelines.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Author's contributions
DMP conceived the study, performed the surgery, and wrote the manuscript. PM and MN assisted with the cadaveric dissections, provided logistical support, and proofread the manuscript. All authors agreed to the final manuscript content. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Lindsay C, Simpson J, Ispoglou S, Sturman SG, Pandyan AD. The early use of botulinum toxin in post-stroke spasticity: Study protocol for a randomised controlled trial. Trials 2014;15:12. [Google Scholar] |
| 2. | Brainin M, Norrving B, Sunnerhagen KS, Goldstein LB, Cramer SC, Donnan GA, et al. Poststroke chronic disease management: Towards improved identification and interventions for poststroke spasticity-related complications. Int J Stroke 2011;6:42-6. [Google Scholar] |
| 3. | Namdari S, Alosh H, Baldwin K, Mehta S, Keenan MA. Shoulder tenotomies to improve passive motion and relieve pain in patients with spastic hemiplegia after upper motor neuron injury. J Shoulder Elbow Surg 2011;20:802-6. [Google Scholar] |
| 4. | Namdari S, Alosh H, Baldwin K, Mehta S, Keenan MA. Outcomes of tendon fractional lengthenings to improve shoulder function in patients with spastic hemiparesis. J Shoulder Elbow Surg 2012;21:691-8. [Google Scholar] |
| 5. | Garland DE, Thompson R, Waters RL. Musculocutaneous neurectomy for spastic elbow flexion in non-functional upper extremities in adults. J Bone Joint Surg Am 1980;62:108-12. [Google Scholar] |
| 6. | Brunelli G, Brunelli F. Selective microsurgical denervation in spastic paralysis. Ann Chir Main 1983;2:277-80. [Google Scholar] |
| 7. | Mikalef P, Power D. The role of neurectomy in the management of spasticity of the upper limb. EFORT Open Rev 2017;2:469-73. [Google Scholar] |
| 8. | Keenan MA. Management of the spastic upper extremity in the neurologically impaired adult. Clin Orthop Relat Res 1988;233:116-25. [Google Scholar] |
| 9. | Aszmann OC, Rab M, Kamolz L, Frey M. The anatomy of the pectoral nerves and their significance in brachial plexus reconstruction. J Hand Surg Am 2000;25:942-7. [Google Scholar] |
| 10. | Muthoka JM, Sinkeet SR, Shahbal SH, Matakwa LC, Ogeng'o JA. Variations in branching of the posterior cord of brachial plexus in a Kenyan population. J Brachial Plex Peripher Nerve Inj 2011;6:1. [Google Scholar] |
| 11. | Dancker M, Lambert S, Brenner E. The neurovascular anatomy of the teres major muscle. J Shoulder Elbow Surg 2015;24:e57-67. [Google Scholar] |
| 12. | Cambon-Binder A, Leclercq C. Anatomical study of the musculocutaneous nerve branching pattern: Application for selective neurectomy in the treatment of elbow flexors spasticity. Surg Radiol Anat 2015;37:341-8. [Google Scholar] |
| 13. | Leclercq C. Selective neurectomy for the spastic upper extremity. Hand Clin 2018;34:537-45. [Google Scholar] |
Fulltext Views
2,829
PDF downloads
1,472





