Translate this page into:
The stages of rehabilitation following motor nerve transfer surgery
Corresponding Author:
Joshua L Hill
Department of Hand Surgery, Peripheral Nerve Injury Service, Birmingham Hand Service, Queen Elizabeth Hospital, Birmingham, B15 2TH
UK
emailjosh@hotmail.co.uk
| How to cite this article: Hill JL, Turner LC, Jones RD, Jimulia DT, Miller C, Power DM. The stages of rehabilitation following motor nerve transfer surgery. J Musculoskelet Surg Res 2019;3:60-68 |
Abstract
Nerve transfer surgery is a reliable technique for restoration of motor function for paralysis resulting from peripheral nerve injury. The donor motor branch or fascicle is selected in proximity to the denervated target, and a tension-free end-to-end nerve coaptation is performed allowing rapid neurotisation and functional restoration. To date, a standardised rehabilitation protocol does not exist. The Birmingham Protocol was developed to enhance communication between surgeons and physiotherapists and to improve patients' understanding of the recovery process. It is a six-phase continuous rehabilitation programme designed to improve the outcomes following motor nerve transfer surgery. The programme was developed in a regional peripheral nerve injury service and has been evaluated over 10 years in >500 motor nerve transfer procedures. The programme is simple to understand and implement, allowing patient engagement and standardisation of treatment by non-specialist physiotherapists in rehabilitation units remote from the regional centre. The phases are described with expected timelines for progression for motor nerve transfers at different sites. Core outcome measures are defined to facilitate multicentre research. It is hoped that this protocol will serve as a framework that can be applied in other centres both in the UK and the international community.Introduction
Nerve transfer is a reliable method of reconstruction following paralysis. The technique was popularised for the reconstruction of brachial plexus injuries where nerve root avulsion precludes a reliable direct anatomical nerve repair.[1],[2],[3],[4] The reliable results of the technique have encouraged peripheral nerve surgeons to adopt motor nerve transfer both as an adjunct to proximal primary nerve repair and as a primary reconstructive technique where patients present late, the primary nerve injury is severe or the surgical bed is unsuitable for a nerve graft. The technique may also be used as a salvage option when a primary nerve reconstruction is not progressing as expected. Motor nerve transfer may be used to restore function when nerves are resected for tumour surgery, in motor radiculopathy with no recovery following decompression, and in cases of paralysis following spinal cord injury.
Nerve transfer involves harvest of an expendable motor nerve branch or a fascicle from within a mixed motor and sensory nerve to transfer to the motor branch of the denervated target muscle using microsurgical coaptation. The donor nerve is identified using nerve stimulation, and the selected nerve or fascicle is isolated in a silastic loop that is used to provide gentle traction to facilitate neurolysis without instrumentation. The donor nerve is transected distally to allow proximal rotation to the recipient target motor branch. The recipient is identified anatomically and stimulated to confirm the absence of functioning motor axons. A proximal neurolysis is completed using a silastic loop before neurotomy and distal rotation.
The two nerve ends are prepared for microsurgical end-to-end coaptation using sutures and a fibrin glue or nerve connectors. The repair should be tension free in the anatomical position. Before coaptation, excess redundancy should be resected from the recipient stump, which brings the coaptation site closer to the target muscle, shortening the time for neurotisation.
There are a number of factors that influence the success of motor nerve transfer surgery. The time to re-innervate the target muscle is directly dependent on the distance the axons must regenerate before reaching the motor end plates. This can be crudely approximated by the distance of the coaptation site to the motor point although there is variation in the length of the intramuscular neural plexus for different muscles. The rate of neural regeneration following end-to-end repair is approximately 1 mm per day. Initially, there is a short lag as new structural materials and organelles are generated before cytoskeleton transport to the growth cone. This lag also increases the further; the donor nerve neurotomy is from the anterior horn cell body. Proximity of the coaptation to the denervated muscle provides strong neurotropic stimulation to the proximal growth cone.
The number of available motor axons in the donor nerve is another important factor for successful re-innervation, and typically at least 30% of the original axon numbers in the undamaged motor branch are required to reach the recipient from the donor nerve. Some axons will be lost via apoptosis following donor neurotomy, and others held up at the repair site. Technical errors at the repair site can result in poor endoneurial tube alignment, and there is current interest in sutureless techniques that provide a more consistent method of nerve coaptation.
Longstanding denervation results in reduced neurotropic stimulation, irreversible fatty deposition within the muscle, plus collapse of the intramuscular neural plexus. This renders a denervated target muscle unresponsive to neurotisation unless re-innervation is complete between 9 and 12 months.
There is strong evidence to support the concept of acute motor nerve transfer surgery from animal experiments that have demonstrated the optimisation of axon regeneration and motor re-innervation through restoration of a freshly denervated muscle using an undamaged donor motor nerve. Results of chronically denervated muscle re-innervation are better using an undamaged motor donor than one where there is a delayed coaptation following a chronic neurotomy.[5],[6],[7] In clinical practice, the upper time limit for successful motor nerve transfer is unknown. Cases of late transfer beyond 12 months may be successful when denervation is incomplete, as seen in motor radiculopathies due to degenerative spondyloarthropathy, and upper motor neurone paralysis following spinal cord injury. This chronic partial denervation results in adaptive increases in motor unit size.[8],[9],[10]
Innovation by peripheral nerve surgeons has resulted in attempts to improve the functional outcome of nerve transfer surgery and at the same time minimise the morbidity of the sacrificed donor nerve. In restoration of shoulder function, greater abduction and external rotation are achieved when a double nerve transfer is performed, providing sufficient donors are available. Combining the spinal accessory to the supra-scapular nerve transfer with a triceps to the anterior division of the axillary nerve transfer can restore supraspinatus, infra-spinatus and deltoid function. Similarly, using a modification of the triceps transfer and targeting the main axillary nerve can re-innervate teres minor, improving external rotation.[11] Using the medial branch to triceps as a donor nerve instead of the long head branch in cases of C5 root injury avoids losing the glenohumeral stabilising effect of the long head of triceps in a patient with cuff and deltoid paralysis. This effect is less important in nerve transfers for isolated axillary nerve palsy, but preservation of the bi-articular function of the long head of triceps improves proprioception. The medial triceps branch also has greater motor axon count and a longer branch length, enabling coaptation of the nerve transfer closer to the denervated deltoid muscle, thereby reducing the innervation time.[12],[13]
Peripheral nerve surgeons may also use nerve transfer surgery as an adjunct to a proximal repair or graft for a key distal target. Re-innervation of a muscle following a proximal nerve graft is subject to the uncertainties of axonal misdirection. A sensory axon may populate a motor endoneurial tube, and conversely, a motor axon may be lost to a sensory branch to the skin. These axons are lost through apoptosis due to a lack of sustained appropriate neurotropic stimulation. Successful motor axon re-innervation to a muscle will be functional when sufficient numbers have reached their targets and are accompanied by appropriate motor afferent re-innervation for control. Within a mixed motor and sensory nerve graft, there are opportunities for axon misdirection, hold-up and apoptosis at both ends of the nerve graft. Motor axon count is outnumbered by the sensory axons. The consequence is unpredictable distal motor re-innervation. One theory is that a selective distal nerve transfer, as an adjunct to a primary nerve graft, could improve the chance of a successful outcome.[14],[15]
Adoption of the technique of motor nerve transfer surgery by a wider group of surgeons with more varied indications requires a standardised approach to rehabilitation and outcomes reporting. A standardised approach allows evaluation of these developments in large, multi-centre, patient datasets.
The Birmingham Rehabilitation Protocol
The protocol was developed to define the stages of rehabilitation necessary for peripheral and central nervous system reorganisation to optimise outcomes following motor nerve transfer surgery. The protocol defines six stages of rehabilitation that are easy to understand and recall, encouraging patient understanding and participation, which are essential for a successful outcome. An additional benefit of the pathway is that it enhances communication with non-specialist physiotherapists leading rehabilitation in peripheral units remote from the tertiary referral nerve centre. An outline of anticipated progression between stages for a given nerve transfer ensures that the correct exercises are introduced at key stages.[16]
The stages of the protocol are not discrete and considerable overlap means that rehabilitation is a continuum for a given transfer. Patients will frequently have more than one nerve transfer to reconstruct a complex peripheral nerve injury, and in such cases, the progression between stages may be different for each transfer.
The stages are defined as pre-operative, protection, prevention, power, plasticity and purpose. The stages are illustrated with reference to the progression expected for a medial triceps to deltoid nerve transfer.
Stage 1: Pre-operative
During this phase, emphasis on patient education and clear communication improves adherence to the rehabilitation protocol. Patients are encouraged to set specific functional goals, which can later be used to measure the outcome of surgery. The concept of nerve transfer surgery is described with emphasis on the specific aspects of the transfer to be undertaken including the donor muscle, recipient function and potential morbidity.[17]
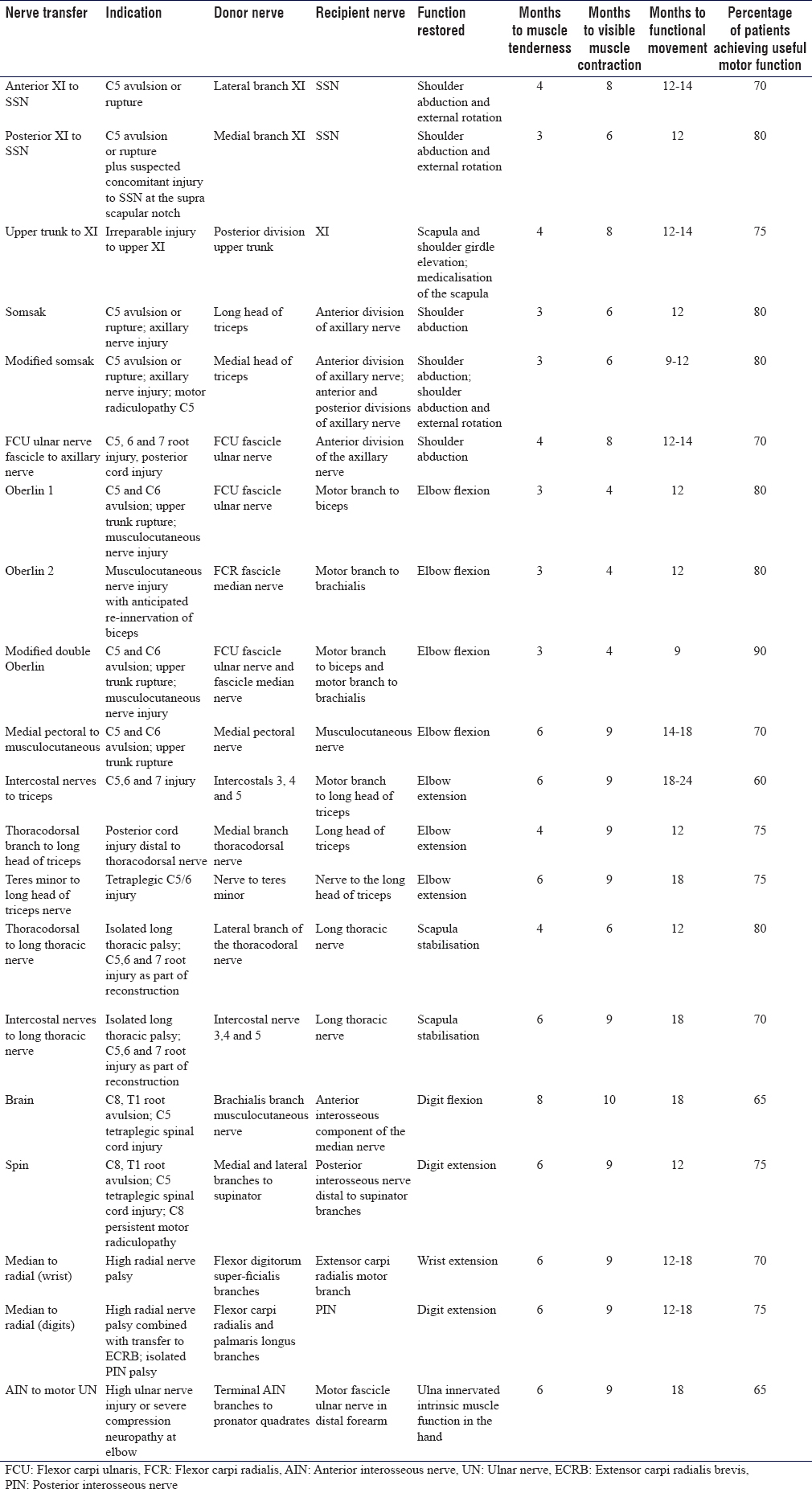
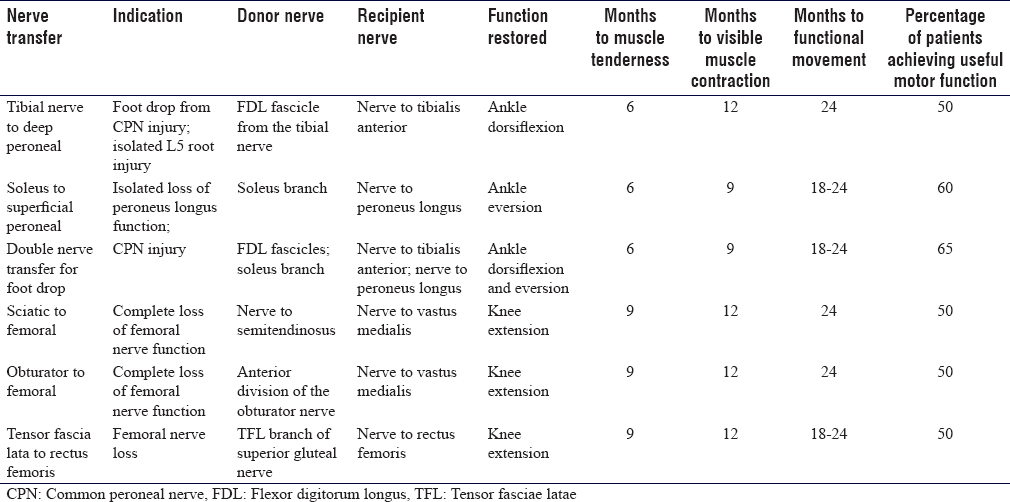
Patients must be aware that following nerve transfer surgery, activation of the newly innervated muscle is variable and unique to them. Estimates of innervation time can be calculated from pooled retrospective patient datasets [Table - 1] and [Table - 2]. However, definite timescales are difficult to predict, and many factors influence the rate and quality of the re-innervation. They should not be disappointed if target muscle re-innervation is not apparent for several months, and exercises should be continued as defined in the protocol to improve the chances of successful transfer.
Following nerve transfer, a new nerve innervates the target muscle; therefore, previously established motor patterns and cortical maps are no longer relevant. It is necessary to establish new motor patterns using the donor muscle through guided motor imagery.
The donor muscle is used throughout the rehabilitation process, and pre-operatively, it must be strengthened. The donor and related muscles in the same group can become weak, hindering recovery. This can be due to disuse atrophy or low-grade associated nerve injury.
Repeated contraction of the donor and visualisation of this contraction ensure that the patient is able to recruit the donor during the post-transfer phase of rehabilitation.[18]
In preparation for a triceps to axillary nerve transfer, the patient will be required to strengthen the triceps through resisted elbow extension exercises. The patient is trained to perform isometric contraction of the triceps to facilitate the continued rehabilitation during stage 2 of the rehabilitation pathway when the shoulder is immobilised.[19],[20]
The patient is encouraged to perform bimanual activation using elbow extension and concomitant shoulder abduction of the contralateral limb and imagery for the ipsilateral deltoid activation using a mirror.
Joint passive range of motion is maintained in preparation for the short period of immobilisation after nerve transfer. The patient is taught passive shoulder abduction and external rotation exercises to prevent shoulder capsule contraction that would otherwise negatively impact the outcome. Weak muscles cannot move a stiff joint, and stiffness impairs functional use of the limb after an otherwise successful nerve transfer. The peak isometric triceps muscle power is recorded using digital myometry in the deep elbow flexion position, mid-range and terminal extension.
The pre-operative stage is typically between 4 and 12 weeks before surgery dependent on the time from injury, need for a period of observation to exclude the possibility of spontaneous recovery, degree of stiffness in the affected joints and degree of wasting of the donor muscle.[18],[19],[20]
Stage 2: Protection
Following surgery, the site of transfer is immobilised to prevent inadvertent disruption of the coaptation site. Isometric contraction of the donor muscle and continued guided motor imagery continues through this phase of rehabilitation. This activity prevents further loss of activity in the remaining donor muscle due to atrophy, maintains electrical stimulation in the transferred branch and reinforces central nervous system motor mapping.
Following a medial branch of triceps to axillary nerve transfer, the operated limb is protected in a poly-sling with a torso strap to prevent excessive passive movements in the shoulder. Triceps is activated with static contraction to maintain function. The activation of the long head assists shoulder relocation and proprioceptive feedback for patients with inferior subluxation after loss of C5 function with supraspinatus and deltoid paralysis.
Joint mobility is maintained at the wrist and digits with active and passive exercises, and the elbow can be extended in the adducted shoulder position regularly to prevent stiffness. The torso strap should be reapplied between these exercises and should continue to be worn full time for 3 weeks after nerve transfer surgery.
During this phase of protection of the operated limb, it is important to continue to develop motor pathways through activation of the required pattern in the contralateral limb (shoulder abduction with elbow extension). Visual imagery is enhanced using a mirror to view the contralateral limb and is coupled with mental imagery of activation on the ipsilateral limb (imagining abducting the affected shoulder with elbow extension).
Stage 3: Prevention
The prevention phase of the rehabilitation protocol continues to activate the donor muscle, and the imagery allows the central reorganisation necessary for activation of the transfer. Protection of the transfer is no longer required, and exercises can be commenced to prevent joint stiffness and to allow neural gliding at the site of surgery.[20] These neural gliding techniques are devised to minimise strain and prevent nerve tethering.[21]
Scar massage can be commenced, and any sensitivity in a mixed nerve territory consequent to highly selective fascicle transfer can be managed with desensitisation and mirror therapy.
Donor muscle atrophy is prevented by strengthening remaining muscle function. Trophic stimulation can be used in cases where there is anticipated adoption of denervated muscle fibres through collateral sprouting from residual motor axons within the muscle. The process of adoption follows a highly selective fascicle transfer where a donor muscle is incompletely denervated and the result is a net increase in motor unit size with restoration of pre-transfer strength and function. Following a nerve transfer where there is complete denervation of a muscle belly by harvesting a complete named motor branch, functional recovery is through improved strength and adaptive spindle changes in the remaining muscle heads.
Following a transfer of the medial triceps branch to the axially nerve, the medial head of triceps and anconeus are paralysed and become wasted. The patient will typically note reduced strength of elbow extension from the deep flexion position with some loss of terminal flexion and proprioceptive awareness. Adaptive changes in the residual lateral and long heads render the functional losses insignificant. In the upright position, gravity assists elbow extension and thus triceps is a useful source of motor axons.[22]
Passive shoulder abduction and external rotation continue with guided motor imagery for central motor mapping. The prevention phase typically lasts around 12 weeks following a transfer. Transition to Stage 4 follows early signs of target re-innervation.
Stage 4: Power
The first sign of potential re-innervation of the target recipient muscle involves a tender muscle on deep palpation.[23] This phenomenon is poorly understood but most likely represents early unmyelinated small fibre autonomic innervation. Small fibres regenerate faster than their larger myelinated motor efferent counterparts. Following a nerve transfer, this is a good prognostic sign and typically visible muscle contractions become apparent between 8 and 12 weeks from onset of muscle tenderness and are potentiated by activation of the donor nerve using mental imagery and co-contraction of residual motor function in the donor territory.
The goals of this stage include further muscle recruitment and strengthening. Gravity-assisted and subsequently gravity-eliminated activation provide visual feedback to patients and allow them to further strengthen the recipient through continued activation of the donor nerve. Auditory bio-feedback through surface electromyographic recording allows exploration of donor activation and maximises the re-innervation activity, training and restoring bulk to the previously paralysed muscle.[24]
Concomitant donor muscle activity can be used to facilitate recipient activation during resisted activation and strengthening; however, ultimately, the goal is to be able to isolate the receipt in isolation without donor recruitment. The timing of progression to stage five is variable and patients must be able to reliably activate their recipient muscle and demonstrate joint movement with gravity eliminated (Medical Research Council [MRC] grade 2) before commencing exercises aimed at improving independent activation of the recipient.
Following a medial triceps to axillary nerve transfer, gravity-eliminated shoulder abduction with simultaneous recruitment of triceps would be performed. This could be achieved initially by the patient lying supine, abducting the shoulder with the elbow extended. Progression from this would involve semi-reclined shoulder abduction with elbow extension, continuing with bilateral activity to maintain visual cortical mapping. The stage 4 duration in a nerve transfer to deltoid is typically around 6 months (9 months from surgery for a triceps to axillary nerve transfer).[4]
Stage 5: Plasticity
The focus on this stage of rehabilitation is to allow further strengthening of the recipient muscle and introduction of exercises to initiate and maintain contraction without overt donor nerve recruitment. Strengthening continues and focus is on increasing peak contraction, endurance and independence.
Automaticity results when the newly innervated muscle contracts without donor muscle activation and can be generated through progressive withdrawal of the donor activation and using biofeedback on the donor muscle group as well as over the recipient.
Following a transfer from the triceps to the deltoid, this phase would continue from 6 months post-transfer to 12 months and be accompanied by progression to MRC grade 3–4 power whereby the patient is able to move against gravity alone or gravity and some resistance. The anti-gravity activation of the deltoid in the upright position commences with elbow extension and graduated shoulder abduction with progressive elbow flexion follows until the deltoid abduction is accomplished without elbow extension. Using myometry, it is typical for the recipient to be stronger with donor activation for 24 months or longer; however, it is the ability to recruit the recipient independently of the donor muscle that demonstrates successful completion of this stage of the rehabilitation protocol.
Stage 6: Purpose
Ultimately, the purpose of motor nerve transfer surgery is to restore function to paralysed muscles, to improve function and to make a meaningful difference to the life of the patient. The individually tailored goals for treatment should be established at the outset and reappraised regularly throughout the course of rehabilitation. The last stage of rehabilitation is to focus on goal attainment through targeted therapy exercises. Although an opportunity to reflect on the subjective success of the procedure and refine activity in terms of control, strength and endurance, the aim of any goal-oriented programme of rehabilitation should include regular evaluation of progress throughout the whole period of rehabilitation.[20] At the end of this stage, a patient should be able to define to what extent their expectations have been achieved, and patient-reported outcomes, goals outcomes and objective motor assessments should be completed for the purpose of audit and research.
Defining a Core Outcome Dataset for Motor Nerve Transfer Surgery
Surgeons and therapists will usually record a muscle power grading using the MRC system, and although this is the most frequent measure of motor nerve transfer success reported in the literature, this is flawed. There is variation in reporting of the MRC with both intra-observer and inter-observer error. The MRC grading also has approximately 85% of possible recorded values falling in a single grade (MRC 4) with a range such that a poor grade 4 power is almost non-functional for many patients and at the other extreme a grade 4 power can be virtually normal function. The grading does not reflect the possibility of muscle fatigue and the lack of endurance, rendering an otherwise good motor outcome poor in terms of patient functional use. Improvements may be achieved through myometry measuring an absolute value, repeated testing and providing a mean score, or through sub-categorisation of MRC grade 4. One such system compares the maximum weight lifted by the recovering limb to the contralateral one. In doing so, grade 4 can be subcategorised into grade 4a (able to lift <30% weight of the normal side), grade 4b (able to lift 30%–60% weight of the normal side) and grade 4c (able to lift >60% weight of the normal side).[25]
The challenge of outcomes measurement, however, is measuring something that is important to an individual patient. Achieving a certain measurable degree of muscle strength or motor activity is not directly related to patient satisfaction or perceived success of the surgery. Generic patient-reported outcomes measures (PROMs) include the Disabilities of the Arm Shoulder and Hand score or the Patient Experience Measure score and offer simplicity for universal application.[26],[27] However, they are not disease specific and often inadequately validated for use in evaluating complex reconstructive surgery. Thus, as a consequence they are a poor alternative method of assessing function or changes in function over time. A paper evaluating patient function after Oberlin transfer demonstrated that MRC grade correlates poorly with function and that improvements in muscle power over time are not reflected in improvements in patient reported outcome scores.[28] Improved disease-specific PROMs may improve outcome reporting and the Brachial Plexus Assessment Tool shows promise.[29]
Perhaps the most obvious way of assessing improvements for an individual patient are goals scores. These are tools used to guide a patient to understand the impact of an injury or disease on their function in recreational, social, domestic and vocational settings. Patients define what is important to them and how difficult the task is at the outset, before intervention and at the completion of treatment, using either a descriptive ranking scale or through assigning a numerical score. The challenge for clinicians is that this process takes time and patients may have unrealistic expectations of their possible outcome. For it to be a valuable experience, a period of guided exploration of key domains is required and then ranking of goals in each sub-domain. The Canadian Occupational Performance Measure is useful as a research tool, but perhaps too cumbersome for use in a regular clinical setting. Alternatively, shortened goal scores may be preferred.[30]
Paralysis results in a loss of function, independence and frequently enforces changes in employment. This may consequently impact financial stability and social interaction. Pain will usually be associated with a nerve injury and chronic pain has significant adverse emotional consequences. Evaluating psychological well-being is not only fundamental to managing patients with paralysis, but it also provides support, encourages engagement and measures response to treatment. There are generic PROMs that measure psychological factors that may influence response to treatment and engagement with rehabilitation. The EuroQol five-dimension test for measuring generic health status is a useful tool that is simple to implement.[31]
Measuring response for an individual patient before and after intervention, or through tracking the effect of intervention over time is extremely valuable, particularly when the timescales for improvement are measured in months and years following motor nerve transfer surgery. They can help to encourage on-going participation in rehabilitation when progress seems slow. The challenge is comparing between individuals and measuring functional improvements between different reconstructive options applied to different cohorts. A numerical value can be assigned to a goal score and used to pool outcomes scores.
Discussion
Motor nerve transfer surgery is established for the reconstruction of paralysis in brachial plexus injury. The technique may be applied to other situations where there is loss of motor function and may be combined with tendon transfer, arthrodesis and free muscle transfer for reconstruction. The advantages of nerve transfer include the restoration of function in the original muscle and no alteration of the resting sarcomere length that inevitably follows tenotomy and re-alignment of a muscle tendon unit when tendon transfer is performed. Rehabilitation after nerve transfer requires muscle re-innervation and then cortical changes to enable independent recruitment of the muscle. Tendon transfers require longer periods of immobilisation and splinting, but useful function is usually achievable more quickly than with nerve transfers, albeit at a greater risk of tendon adhesions that may reduce effectiveness of the transfer. Arthrodesis may provide stability and free up tendons or donor motor nerves for transfer, enabling greater functional gains for a complex case with extensive paralysis. Free muscle transfer requires neurotisation but is reserved for cases where a longstanding paralysis precludes re-innervation of the original muscle or in situ ations where a dual function can be achieved with a single muscle and tendon unit such as the restoration of elbow flexion and wrist extension after total brachial plexus injury.
Conclusion
Motor nerve transfer surgery is a reliable method of restoration of function in the paralysed upper limb. The technique may be applied to different conditions that result in paralysis.
There is no consensus on rehabilitation or outcomes data collection for motor nerve transfer surgery. High-quality, relevant and comparative data are required to measure outcomes and define new treatment pathways.
The Birmingham Protocol is a six-stage continuum for the rehabilitation and evaluation of outcome after motor nerve transfer surgery that addresses both peripheral and central nervous system changes following surgery.
The protocol is simple to understand and can be applied by non-specialist physiotherapists with varied timelines for motor nerve transfer reconstructions with different indications in all anatomical locations.
The protocol must be evaluated and a core outcome dataset to standardise outcomes assessment has been suggested, including measures of muscle strength and endurance in the donor and recipient muscles, PROMs, psychological evaluation and patient-specific goals attainment scores.
Ethical considerations
The paper is a review of current practice and as such no ethics board review was necessary. The project was registered as an audit of outcome in line with local audit and clinical governance requirements at our institution.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Author's contribution
JLH produced the first draft of the article and was responsible for submission and correspondence. LCT edited the first draft and assisted with referencing. RDJ assisted in producing the first draft, referencing, reviewing the article and logistical support. CM guided development of the pathway and the concept for the paper, provided guidance on the stages of the pathway and the importance of physiotherapy strategies. CM was also involved in editing and reviewing the final draft. DMP developed the pathway and conceptualised the paper, outlined the structure, co-authored the first draft, edited, reviewed and referenced the final paper. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Oberlin C, Béal D, Leechavengvongs S, Salon A, Dauge MC, Sarcy JJ, et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: Anatomical study and report of four cases. J Hand Surg Am 1994;19:232-7. [Google Scholar] |
| 2. | Teboul F, Kakkar R, Ameur N, Beaulieu JY, Oberlin C. Transfer of fascicles from the ulnar nerve to the nerve to the biceps in the treatment of upper brachial plexus palsy. J Bone Joint Surg Am 2004;86-A: 1485-90. [Google Scholar] |
| 3. | Witoonchart K, Leechavengvongs S, Uerpairojkit C, Thuvasethakul P, Wongnopsuwan V. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, Part I: An anatomic feasibility study. J Hand Surg Am 2003;28:628-32. [Google Scholar] |
| 4. | Leechavengvongs S, Witoonchart K, Uerpairojkit C, Thuvasethakul P. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, Part II: A report of 7 cases. J Hand Surg Am 2003;28:633-8. [Google Scholar] |
| 5. | Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged axotomy. J Neurosci 1995;15:3876-85. [Google Scholar] |
| 6. | Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged denervation. J Neurosci 1995;15:3886-95. [Google Scholar] |
| 7. | Holmes WY, Young JZ. Nerve regeneration after immediate and delayed suture. J Anat 1942;77(Pt 1):63. [Google Scholar] |
| 8. | Fox IK. Nerve transfers in tetraplegia. Hand Clin 2016;32:227-42. [Google Scholar] |
| 9. | Mackinnon SE, Yee A, Ray WZ. Nerve transfers for the restoration of hand function after spinal cord injury. J Neurosurg 2012;117:176-85. [Google Scholar] |
| 10. | Brown JM, Mahan MA, Mandeville R, Carter BS. Establishing reconstructive neurosurgery as a subspecialty. Neurosurg Focus 2017;43:E7. [Google Scholar] |
| 11. | Merrell GA, Barrie KA, Katz DL, Wolfe SW. Results of nerve transfer techniques for restoration of shoulder and elbow function in the context of a meta-analysis of the English literature. J Hand Surg Am 2001;26:303-14. [Google Scholar] |
| 12. | Mackinnon SE, Colbert SH. Nerve transfers in the hand and upper extremity surgery. Tech Hand Up Extrem Surg 2008;12:20-33. [Google Scholar] |
| 13. | Colbert SH, Mackinnon S. Posterior approach for double nerve transfer for restoration of shoulder function in upper brachial plexus palsy. Hand (N Y) 2006;1:71-7. [Google Scholar] |
| 14. | Baltzer H, Woo A, Oh C, Moran SL. Comparison of ulnar intrinsic function following supercharge end-to-side anterior interosseous-to-ulnar motor nerve transfer: A Matched cohort study of proximal ulnar nerve injury patients. Plast Reconstr Surg 2016;138:1264-72. [Google Scholar] |
| 15. | Bertelli JA, Soldado F, Rodrígues-Baeza A, Ghizoni MF. Transfer of the motor branch of the abductor digiti quinti for thenar muscle reinnervation in high median nerve injuries. J Hand Surg Am 2018;43:8-15. [Google Scholar] |
| 16. | Escudero RB, Rezende MR, Wataya EY, Pontes FV, Cho ÁB, Pisani MJ, et al. Correlation between the elbow flexion and the hand and wrist flexion after neurotization of the fascicles of the ulnar nerve to the motor branch to the biceps. Rev Bras Ortop 2017;52:309-14. [Google Scholar] |
| 17. | Stevens A, Köke A, van der Weijden T, Beurskens A. Ready for goal setting? Process evaluation of a patient-specific goal-setting method in physiotherapy. BMC Health Serv Res 2017;17:618. [Google Scholar] |
| 18. | Kahn LC, Moore AM. Donor activation focused rehabilitation approach: Maximizing outcomes after nerve transfers. Hand Clin 2016;32:263-77. [Google Scholar] |
| 19. | Novak CB, von der Heyde RL. Evidence and techniques in rehabilitation following nerve injuries. Hand Clin 2013;29:383-92. [Google Scholar] |
| 20. | Novak CB. Rehabilitation following motor nerve transfers. Hand Clin 2008;24:417-23, vi. [Google Scholar] |
| 21. | Silverman PM, Gordon L. Early motion after replantation. Hand Clin 1996;12:97-107. [Google Scholar] |
| 22. | Kholinne E, Zulkarnain RF, Sun YC, Lim S, Chun JM, Jeon IH, et al. The different role of each head of the triceps brachii muscle in elbow extension. Acta Orthop Traumatol Turc 2018;52:201-5. [Google Scholar] |
| 23. | Lee EY, Karjalainen TV, Sebastin SJ, Lim AY. The value of the tender muscle sign in detecting motor recovery after peripheral nerve reconstruction. J Hand Surg Am 2015;40:433-7. [Google Scholar] |
| 24. | Sturma A, Hruby LA, Prahm C, Mayer JA, Aszmann OC. Rehabilitation of upper extremity nerve injuries using surface EMG biofeedback: Protocols for clinical application. Front Neurosci 2018;12:906. [Google Scholar] |
| 25. | Bhardwaj P, Bhardwaj N. Motor grading of elbow flexion-is Medical Research Council grading good enough? J Brachial Plex Peripher Nerve Inj 2009;4:3. [Google Scholar] |
| 26. | Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder and hand) [corrected]. The upper extremity collaborative group (UECG) Am J Ind Med 1996;29:602-8. [Google Scholar] |
| 27. | Macey AC, Burke FD, Abbott K, Barton NJ, Bradbury E, Bradley A, et al. Outcomes of hand surgery. J Hand Surg 1995;20:841-55. [Google Scholar] |
| 28. | Brown H, Johnson K, Gilbert A, Quick TJ. The lived experience of motor recovery of elbow flexion following Oberlin nerve transfer: A qualitative analysis. Hand Therapy 2018;23:130-8. [Google Scholar] |
| 29. | Hill B, Pallant J, Williams G, Olver J, Ferris S, Bialocerkowski A, et al. Evaluation of internal construct validity and unidimensionality of the brachial assessment tool, a patient-reported outcome measure for brachial plexus injury. Arch Phys Med Rehabil 2016;97:2146-56. [Google Scholar] |
| 30. | Law MC, Baptiste S, Carswell A, McColl MA, Polatajko H, Pollock N. Canadian Occupational Performance Measure: COPM. CAOT Publ., ACE; 1998. [Google Scholar] |
| 31. | EuroQol Group. EuroQol – A new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. [Google Scholar] |
Fulltext Views
16,565
PDF downloads
3,528





