Translate this page into:
An unusual presentation of a malignant transformation in a recurrent solitary clavicle osteochondroma
2 Department of Orthopedics, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
Corresponding Author:
Aliaa F Khaja
Department of Orthopedics, Alrazi Orthopedic Hospital, Kuwait Institution for Medical Specializations, Kuwait City
Kuwait
aliaa.khaja@gmail.com
| How to cite this article: Khaja AF, Alshammari AN, Abdelmotaal MM. An unusual presentation of a malignant transformation in a recurrent solitary clavicle osteochondroma. J Musculoskelet Surg Res 2018;2:167-172 |
Abstract
Osteochondromas are characteristically benign cartilaginous neoplasms and are one of the most frequent benign tumors, usually targeting the axial skeleton. We came across an unusual presentation of this lesion, whereby the location of the osteochondroma is unique to the literature, and the morphological features are classified as rare. This study reviewed a case of a resected osteochondroma in a clavicle, which reoccurred a year later as chondrosarcoma. The decision was made to resect the malignant transformation. Due to the critical location of the lesion, the clavicle was left in a position where it was suspended. To protect the anatomical surroundings from the suspended clavicle, a pectoralis major flap was sutured to the sternocleidomastoid muscle. The patient has been symptom free for the past 2 years, and functional abilities have been preserved.
Introduction
The clavicle is a rare site for bone tumors, with the majority of reported cases being malignant in nature. The major pathology differentials include eosinophilic granuloma, plasmacytoma, Ewing sarcoma, osteosarcoma, osteochondroma, chondrosarcoma, and aneurysmal bone cysts.[1]
Osteochondromas are benign bony protrusions with cartilaginous caps. They are established as the second-most common benign bone tumors after nonossifying fibromas, mainly affecting the bones of the axial skeleton, more specifically around the knee joint.[2] However, they can occur elsewhere, although this is an unusual presentation. Unni,[3] reported that chondrosarcomas are the second-most common bone tumors after osteosarcoma, constituting 9.2% of cases in the Mayo Clinic series of 11,087 cases.
The cartilaginous plate grows outwards instead of longitudinally, forming an osteochondroma rendering it to form a bony lesion covered with a cartilage cap. Osteochondromas are frequently incidentally found while investigating other complaints and are commonly asymptomatic. If symptomatic, with pain, however, it would be due to the mechanical compression, on the overlying soft-tissue structures, such as muscles, tendons, and nerves.[4] In some rare cases of osteochondromas, compressive myopathy has been documented.[4],[5]
Although osteochondromas are considered benign tumors, chondrosarcomas are considered to be cartilage forming malignant tumors. The preexistence of osteochondromas is the main differentiation between primary and secondary chondrosarcomas. Primary chondrosarcoma, on the other hand, does not arise from a preexisting lesion.[6]
Secondary chondrosarcoma is not always easy to diagnose, and the histologic features alone may not be sufficient to determine that a lesion has become malignant. The current literature provided a list of specific risk factors, which can be used to help the diagnostic suspicions of secondary chondrosarcoma. These factors are grouped into clinical, radiological, and genetic. Clinical factors include pain along with an increasing size of a palpable lesion, male predominance (2:1), the location of pelvis or hip as well as a peak age in mid-30 s. Radiographic risk factors consist of surface integrity, blurriness of the border, osteochondroma >5 cm and cartilage cap thickness of >2 cm and inhomogeneous mineralization of large cartilage cap. The genetic group of risk factors comprised of the predisposition of genetic mutations in EXT1, EXT2, EXT3, which are found in patients with hereditary multiple osteochondroma (estimated 2%–4% risk), or diseases such as Ollier disease and Maffucci syndrome (estimated 10%–40% risk).[6]
There is notably a cortical bony continuation with the possibility of accumulating a variety of calcifications within the cartilage cap.[7] The risk of malignant transformation of benign cartilage tumors has not been well established, and according to Lin et al.,[6] the risk for solitary osteochondromas is most likely <1%.[7]
Malignant transformation is a counterpart of osteochondromas, and when the transformation occurs, it is classified as a separate entity named secondary chondrosarcoma. Should there be a relative thickness of the cartilage cap present (>2 cm), one should consider the probability of the presence of a malignant transformation, but there is no established cutoff point for the size of the thickness of the cartilage cap. Nevertheless, in general, an increased cartilage cap thickness of >2 cm is a strongly suggestive evidence of the presence of secondary chondrosarcoma.[3] Although one must emphasize that this is a somewhat rare phenomenon, and almost exclusively seen in adults and has a good prognosis.[3],[7]
In the literature, it was found that the clavicular osteochondromas account for 8.7% of the 206 cases observed in East Asia, with malignancy still a rare finding, and noted to increase above the age of 40.[1],[8]
We are reporting this case to investigate this curious case of reoccurrence, and to provide input of our surgical outcome with a comparison to the currently suggested treatments.
Case Report
A 37-year-old man, from Bangladesh, that was previously well, presented with a year history of point tenderness over the medial aspect of his right clavicle, and a notable hard swelling. The swelling had been present for 10 years and was characterized by a gradual increase in size over the years. He reported pain that was interfering with his activities of daily living. He denied having dyspnea, weight loss, anorexia, night sweats, or fever. At first presentation, the swelling was hard on palpation and approximately 3 cm × 4 cm in size, mildly tender and the overlying skin was mobile. There were no neurological deficits in the right upper limb. The radiographs showed a bony lesion protruding from and continuous with the underlying bone of the medial third of the right clavicle [Figure - 1]. The magnetic resonance imaging (MRI) was done in his home country; the limited number of cuts provided was not enough for proper measurement. A repeat of initial MRI in the treating facility was unobtainable as the patient had financial limitations [Figure - 2]. Subsequently, a computed tomography (CT) scan was done and showed a mass that measures 2.2 cm × 2.2 cm × 2.5 cm, with a cauliflower-like pattern, a short, small stalk with the medulla and cortex being continuous with the native clavicle bone. The lesion had a cartilage cap without calcifications. A step-wise treatment with analgesics was initiated without adequate relief. The mass was then excised. Postoperative complications were unremarkable, and the recovery was uncomplicated. The biopsy result was in keeping with an osteochondroma. It also showed that there were multiple bony fragments encompassed by a cartilage cap 1 cm thick in maximum thickness. The largest of these fragments measure around 3.0 cm × 3.4 cm × 2.0 cm.
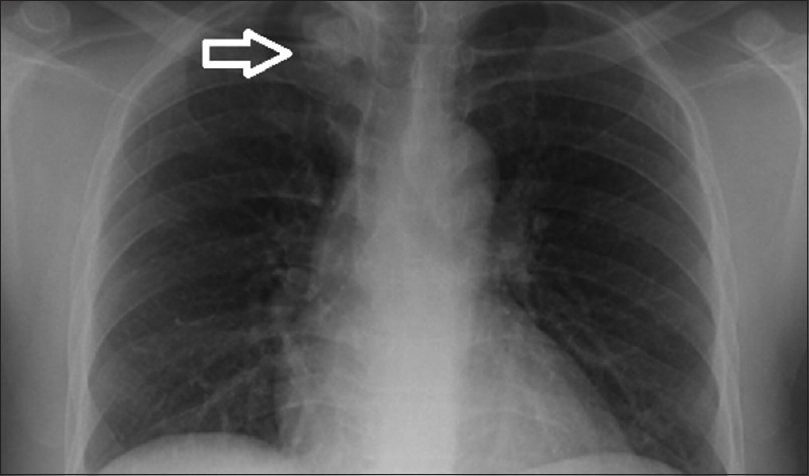 |
| Figure 1: Chest radiograph, showing a lesion (arrow) on the medial 3rd of the right clavicle |
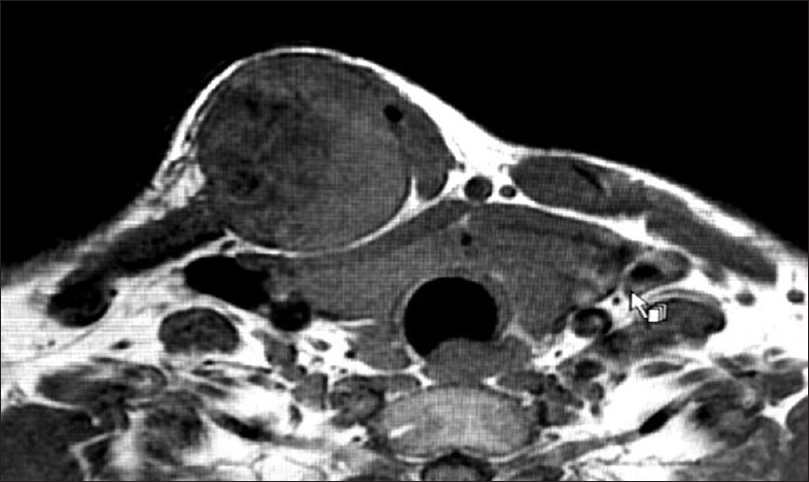 |
| Figure 2: Magnetic resonance imaging of an initial tumor |
Precisely, 1 year later in the same facility, it was noted that the patient had suffered from a reoccurrence of the mass at the site of the previous incision, the pain had returned mirroring the initial presentation, and the size was increasing noticeably in the past year. His range of motion remained unchanged. He denied having any constitutional symptoms. On examination, there was a hard-palpable tender mass, approximately 4 cm × 4 cm in size. The radiographs, CT and MRI in the 2nd presentation, showed an ill-defined solitary opacity in the medial third of the right clavicle, with irregular margins and tiny dots of calcifications within the lesion, suggestive of a sarcomatous transformation [Figure - 3] and [Figure - 4].
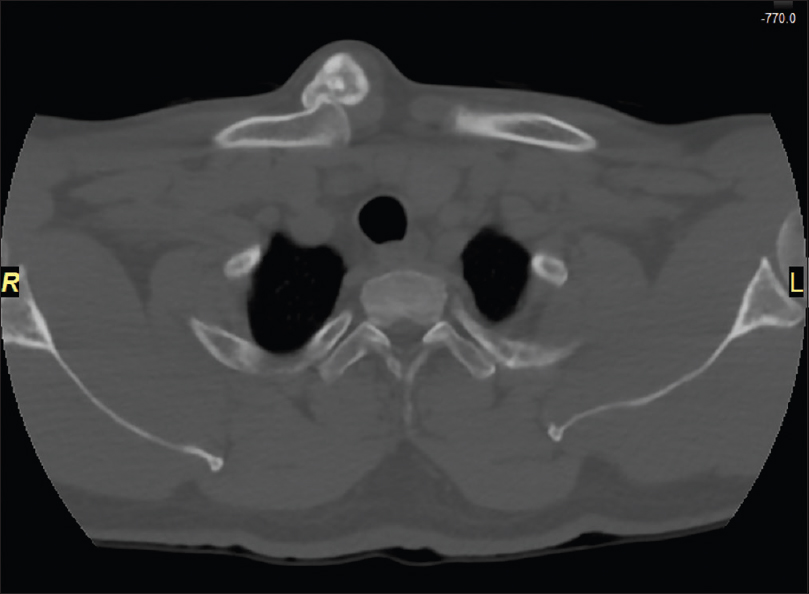 |
| Figure 3: Axial view computed tomography scan of the reoccurring tumor |
 |
| Figure 4: Magnetic resonance imaging of a reoccurring tumor |
A CT-guided biopsy confirmed that the mass had characteristic features of malignant transformation, it had shown differentiated cartilaginous tissue merging with chondromyxoid areas containing spindle-shaped cells, as well as foci showing chondrocyte necrosis.
The patient underwent another surgery to excise the mass (wide surgical resection was performed, with a thoracic surgeon present). The decision for surgery was aimed at three main purposes, a safe oncological resection of the malignancy, avoiding possible structural damage to surrounding anatomy that could compromise the patient's breathing, and reliving the pain.
Under general anesthesia, with the patient in a supine position, an elliptical, transverse incision in the neck was done over the previous surgery scar. Another consecutive long midline incision, starting from the medial edge of the mass, going over the sternum, and until the end of the manubrium to create a flap [Figure - 5]. The right sternocleidomastoid (SCM) muscle was identified and preserved with its deep anatomical relations. The SCM muscle was in turn split at its middle part, with parts of the strap muscles. Superficial dissection followed, exposing the subclavian and jugular veins. The sternum was divided in the midline approximately 2 cm near the addressed tumor. Then the clavicle was detached 2 cm away medially from the mass, with a part of the sternum and the sternoclavicular joint (SCJ). Laterally, it was detached from the junction of the middle and lateral third of the clavicle. After dissection of the tumor, the partial pectoralis major muscle was used as an advancement flap reconstruction and fastened to the SCM muscle.
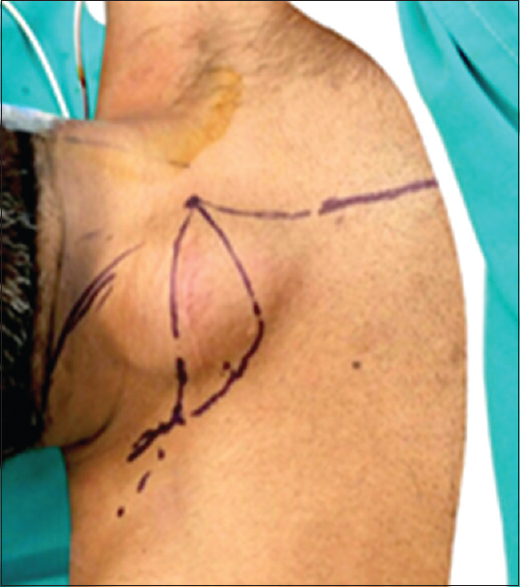 |
| Figure 5: Anatomical landmarks before resection |
The patient had an uncomplicated recovery immediately postoperative, neither breathing difficulties were observed nor neurovascular deficits, and the right upper limb was initially immobilized in a broad-arm sling. Although he had limited right shoulder abduction, which then gradually improved during his postoperative stay after adequate pain relief was provided during his recovery period before discharge [Figure - 6]. An affirmative histological report of the excisional biopsy taken after the reoccurrence showed that the lesion was 5.5 cm × 4 cm × 3 cm in size; the margins of the wide local resection were negative. These findings were compatible with the diagnosis of chondrosarcoma Grade 2 with no bony invasion [Figure - 7].
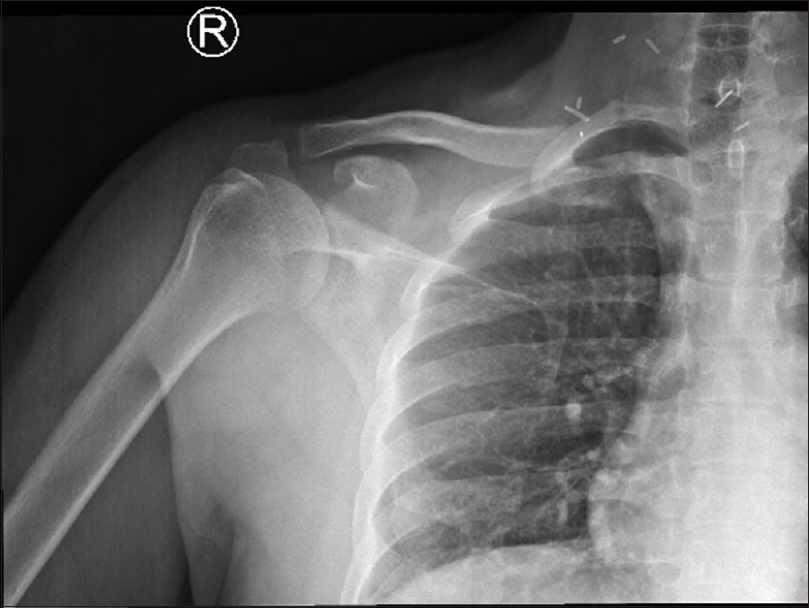 |
| Figure 6: Postoperative radiograph anteroposterior view, after the 2nd resection |
 |
| Figure 7: A reoccurring tumor (Surgical Specimen) |
Four months after surgery, the patient achieved a full range of motion level in his right shoulder, and there was no shortening of the right limb compared to the left limb. No neurological deficits were noted, nor were any functional disabilities recognized. The mass was no longer palpable.
Inflammatory markers were within the normally expected limits. These clinical features remained consistent under our close follow-up for the next 2 years. The recent CT scan [Figure - 8] showed no evidence of visible masses confirming that the lung fields were clear and no visible nodules or masses were seen.
 |
| Figure 8: No evidence of recurrence was found in the follow-up computed tomography scan at 2 years |
Discussion
It was unusual to observe an osteochondroma outside the axial skeleton initially as it is a rare entity. Witnessing a reoccurrence in 1 year is an alarming indication that demands assessment. In our case, the patient had risk factors leading to the suspicion of secondary chondrosarcoma, which was demonstrated by the age and male gender of the patient. He also hails from East Asia; he was treated for an osteochondroma in his right clavicle. One year after the excision of the osteochondroma, he presented again, with the complaint of a new onset of pain and increasing mass of more than 5 cm, surface irregularity, blurred lesion borders on radiographs and the repeat MRI reported a cartilage cap thickness of 1 cm, although it is lower than the >2 cm cartilaginous cap suggested as strong evidence in the current guidelines, we went ahead investigating the nature of this new suspicious lesion (based on the risk factors) by doing a CT-guided biopsy as per standard investigations conducted when suspecting such lesions, radiographs, CT, and MRI images are a must for bony and soft-tissue structure assessment.
The tumor was classified histologically using the criteria of Evans et al.[9] Once the biopsy histopathology report revealed transformation characteristics, it was decided that staging the tumor will aid the treatment decisions.[10] For instance, it has been noted that the majority of secondary chondrosarcomas reported in the literature are either Grade 1 or Grade 2 with a mere 1% constituting Grade 3.[3] It was reported that more than three-fourths of the cases were Grade 1 tumors, and nearly all of the remaining lesions were Grade 2.[6],[7],[11],[12],[13] In our case, the tumor was Grade 2 with symptoms of pain, mostly at night. It is worth mentioning that perhaps a residual or incomplete excision of the initial osteochondroma due to the limitations of the location, which eventually led to the reoccurrence with a malignant transformation, had there been a complete resection of the initial lesion, there would not have been a malignant reoccurrence, or it could have been a chondrosarcoma from the beginning and was missed by the pathologist the 1st time.
The treatment options for secondary chondrosarcoma are currently – to our best knowledge – wide surgical resection. In general, chemotherapy and radiotherapy are not effective with secondary chondrosarcoma. Marginal excisions are witnessed to be at high risk for recurrence. The option of amputation is uncommon in primary surgeries, including large pelvic tumors, yet they become common after local recurrence.[2] The decision was made to abide by the current treatment guidelines, and a wide local excision was done accordingly.
Asymptomatic osteochondroma patients are usually advised for the conservative route whereby symptomatic neoplasms or malignant transformations are recommended to undergo surgery with wide-margin excision in both adults and pediatric patients. As indicated by the literature, the decision to perform the exact surgical technique is inconclusive. Meaning surgical approaches and techniques can differ but provide the same outcomes, whether it be an open surgery or arthroscopic. However, there is a general consensus that the cartilage cap must be resected with the base of the stalk to avoid reoccurrence.[14],[15]
Chest wall and pelvic chondrosarcomas have been previously reported, with oncological site outcomes limited to chondrosarcomas arising in the scapular region.[16] It has been assumed that patients who had chondrosarcoma of the scapula have better treatment outcomes than patients who have chondrosarcomas arising in the other flat bones.[16] This is attributed to the fact that chondrosarcomas are treated with surgical resection, as it is easier to access the scapula due to its superficial location. Whereas other flat bones in the pelvis or chest wall, have vital organs in close proximity limiting the surgical resection. For example, the ribs and clavicles are near structures such as the lung, brachial plexus, and axillary blood vessels, despite the location to such vital structures, which were rarely compromised by the tumors present in this location.[16] There is only one report describing a clavicular tumor causing thoracic outlet syndrome,[17] and only one report of secondary chondrosarcoma causing thoracic outlet syndrome, to our knowledge.[18]
In our report, the diagnosed chondrosarcoma did not cause thoracic outlet syndrome, as the clinical picture, as well as the radiographic images, did not suggest that the tumor was confined mainly to the clavicle, and not invading or compressing the first rib, the subclavius muscle nor the anterior and posterior scalene muscles. Yet the surgeons were aware and cautious regarding this particularly delicate surgical area with regards to the limitations reflected in the underlying vital structures.
A wide surgical excision of the medial part of the clavicle and SCJ with pectoralis major flap coverage of the defect provides soft-tissue coverage of underlying structures, including the major vessels, and is important in cosmesis, with remarkably little disability.[19],[20] In our case, due to the delicate position of this malignant reoccurrence in the clavicle region, a partial pectoralis major advancement flap was constructed to fill the resulting defect and to protect the deeper structures after tumor resection, without functional compromise.
As previously mentioned the current recommended disease-free survival period is 10 years as opposed to the standard 5 years. Hence, the authors concur that this case report will serve as an interim report, as they intend to follow-up the patient for the remainder of this suggested period, with the intent to collaborate with the orthopedic oncology team if he returns to his native country before reaching the set period at hand.
Conclusion
It was important to document this case as this is a rare location for osteochondroma occurrences, to begin with, and it is even rarer to see a secondary transformation into chondrosarcoma in this region. It has been an eye-opener to increase one's risk threshold for such entities further.
Surgical management by tumor excision and partial pectoralis major advancement flap reconstruction is a viable option in such cases and can be performed safely with good outcome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Authors' contributions
AFK and ANA conceived and designed the study. AFK and MMA collected and organized data while AFK and ANA wrote initial and final draft of the article and provided critical input. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Ren K, Wu S, Shi X, Zhao J, Liu X. Primary clavicle tumors and tumorous lesions: A review of 206 cases in East Asia. Arch Orthop Trauma Surg 2012;132:883-9. [Google Scholar] |
| 2. | Saglik Y, Altay M, Unal VS, Basarir K, Yildiz Y. Manifestations and management of osteochondromas: A retrospective analysis of 382 patients. Acta Orthop Belg 2006;72:748-55. [Google Scholar] |
| 3. | Unni KK. Chondrosarcoma (Primary, Secondary, Dedifferentiated, and Clear Cell), in Dahlin's Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA, Lippincott-Raven; 1996. p. 71-108. [Google Scholar] |
| 4. | Mnif H, Koubaa M, Zrig M, Zammel N, Abid A. Peroneal nerve palsy resulting from fibular head osteochondroma. Orthopedics 2009;32:528. [Google Scholar] |
| 5. | Veeravagu A, Li A, Shuer LM, Desai AM. Cervical osteochondroma causing myelopathy in adults: Management considerations and literature review. World Neurosurg 2017;97:752.e5-752.e13. [Google Scholar] |
| 6. | Lin PP, Moussallem CD, Deavers MT. Secondary chondrosarcoma. J Am Acad Orthop Surg 2010;18:608-15. [Google Scholar] |
| 7. | Ostensen H, Pettersson H, Davies AM. The World Health Organization Manual of Diagnostic Imaging: Radiographic Anatomy and Interpretation of the Musculoskeletal System. World Health Organization; 2002. Available from: http://www.apps.who.int/iris/bitstream/10665/42457/1/9241545550_eng.pdf?ua=1. [Last accessed on 2017 Oct 29]. [Google Scholar] |
| 8. | Staals EL, Bacchini P, Mercuri M, Bertoni F. Dedifferentiated chondrosarcomas arising in preexisting osteochondromas. J Bone Joint Surg Am 2007;89:987-93. [Google Scholar] |
| 9. | Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: A clinicopathologic analysis with emphasis on histologic grading. Cancer 1977;40:818-31. [Google Scholar] |
| 10. | Raswan US, Bhat AR, Tanki H, Samoon N, Kirmani AR. A solitary osteochondroma of the cervical spine: A case report and review of literature. Childs Nerv Syst 2017;33:1019-22. [Google Scholar] |
| 11. | Fageir MM, Edwards MR, Addison AK. The surgical management of osteochondroma on the ventral surface of the scapula. J Pediatr Orthop B 2009;18:304-7. [Google Scholar] |
| 12. | Ohnishi T, Horii E, Shukuki K, Hattori T. Surgical treatment for osteochondromas in pediatric digits. J Hand Surg Am 2011;36:432-8. [Google Scholar] |
| 13. | Kim JI, Kwon JH, Park YJ, D'Almeida VR, Soni SM, Nha KW, et al. Arthroscopic excision of solitary intra-articular osteochondroma of the knee. Knee Surg Relat Res 2013;25:36-9. [Google Scholar] |
| 14. | Nogueira Drumond JM. Efficacy of the enneking staging system in relation to treating benign bone tumors and tumor-like bone lesions. Rev Bras Ortop 2010;45:46-52. [Google Scholar] |
| 15. | Di Giorgio L, Lanzone R, Sodano L, Di Paola B, Touloupakis G, Mastantuono M, et al. Surgical treatment of osteochondromas: Indication in “Strategic exostosis”. Clin Ter 2015;166:e27-33. [Google Scholar] |
| 16. | Pant R, Yasko AW, Lewis VO, Raymond K, Lin PP. Chondrosarcoma of the scapula: Long-term oncologic outcome. Cancer 2005;104:149-58. [Google Scholar] |
| 17. | Mollano AV, Hagy ML, Jones KB, Buckwalter JA. Unusual osteochondroma of the medial part of the clavicle causing subclavian vein thrombosis and brachial plexopathy. A case report. J Bone Joint Surg Am 2004;86-A: 2747-50. [Google Scholar] |
| 18. | Kobayashi H, Ikegami M, Ushiku T, Anraku M, Ohki T, Shinoda Y, et al. Secondary chondrosarcoma presenting with symptoms similar to thoracic outlet syndrome. Case Rep Orthop 2018;2018:9347145. [Google Scholar] |
| 19. | Song HK, Guy TS, Kaiser LR, Shrager JB. Current presentation and optimal surgical management of sternoclavicular joint infections. Ann Thorac Surg 2002;73:427-31. [Google Scholar] |
| 20. | Zehr KJ, Heitmiller RF, Yang SC. Split pectoralis major muscle flap reconstruction after clavicular-manubrial resection. Ann Thorac Surg 1999;67:1507-8. [Google Scholar] |
Fulltext Views
4,412
PDF downloads
1,265





