Translate this page into:
Failed carpal tunnel surgery: A guide to management
2 The Hand Unit, Queen Elizabeth Hospital Birmingham; West Midlands Brachial Plexus and Peripheral Nerve Injury Service, Birmingham, UK
Corresponding Author:
Martin Van
The Hand Unit, Queen Elizabeth Hospital Birmingham, Birmingham
UK
martinmtvan@gmail.com
| How to cite this article: Van M, Jose RM, Power D. Failed carpal tunnel surgery: A guide to management. J Musculoskelet Surg Res 2019;3:30-39 |
Abstract
Revision surgery constitutes up to 2.7% of cases of carpal tunnel surgery in the UK. Failed carpal tunnel surgery can present as deterioration, recurrence or persistence of symptoms after surgical decompression. The causes of failed carpal tunnel decompression can often be categorised into four groups; poor surgery, poor nerve, poor diagnosis or poor luck. This situation calls for a structured review of the clinical history, examination, previous investigations and subsequently devising a management plan. We reviewed relevant articles on PubMed, Medline, Embase and Ovid, and we provided a structured approach to failed carpal tunnel surgery based on the current evidence. There are several options for revision carpal tunnel surgery that can be implemented to protect the median nerve, including local flaps, collagen and synthetic polymer wraps. A majority of patients experience improvement in symptoms after revision surgery.
Introduction
Carpal tunnel syndrome (CTS) is the most common peripheral compression neuropathy, and surgical treatment with carpal tunnel decompression (CTD) is one of the most common hand operations performed.[1] The prevalence of CTS is 7%–16% in the United Kingdom (UK) with a rate of 43–47 per 100,000 undergoing CTD surgery.[2],[3] Approximately 2.7% of CTD operations in the UK are revision procedures due to recurrent, persistent or new median nerve symptoms, and these are technically more difficult to perform due to diagnostic uncertainty and scarring of the tissues.[4] Failed CTD occurs when there is persistent, recurring or new carpal tunnel symptoms, and the treating clinician should be aware of the potential reasons for a suboptimal outcome and employ a structured approach to subsequent assessment, investigation, diagnosis and management.
Materials and Methods
Articles were searched in PubMed, Medline, Embase and Ovid databases regarding revision carpal tunnel surgery. Keywords included carpal tunnel surgery, carpal tunnel revision, carpal tunnel decompression, carpal tunnel release and CTS. Articles in English were included, and any relevant article by their title had their abstract was screened. References in the article were also included if relevant and not previously found on the primary search.
Clinical Presentation
CTS usually presents with intermittent paraesthesia, and most patients describe a diurnal variation with nocturnal symptoms, resulting in sleep disturbance or symptoms on wakening. Other common symptoms include pain in the hand or wrist and persistent paraesthesia, progressive weakness and numbness. Paralysis of the intrinsic thumb muscles supplied by the median nerve can also develop, usually late in the syndrome, resulting in loss of power and precision grip, making it difficult for patients to hold a jar or pick up small objects.
Diagnosis of Carpal Tunnel Syndrome
The clinical diagnosis of CTS is based on the patient's history and examination findings. On examination, patients may have a positive Tinel's test over the volar wrist, a positive Phalen's test and reproduction of symptoms on compression testing. The diagnostic accuracy of these clinical tests has been widely studied but variations in methodology, quality of study design and control groups; the literature has quoted a wide range of sensitivity and specificity.[5] A systematic review by MacDermid and Wessel calculated the average diagnostic sensitivity and specificity of high-quality studies to be 68% and 73% for Phalen's test, 50% and 77% for Tinel's test and 64% and 83% for carpal compression test, respectively. Combining these clinical tests improves diagnostic accuracy.[6]
In terms of investigations, nerve conduction studies (NCSs) can be an useful objective measure in diagnosing CTS.[7] Patients with CTS will characteristically have reduced amplitude studies, reduced velocity of NCSs and increased latency on neurophysiological studies. In cases with longstanding and severe compression, there will be evidence of denervation on electromyographic (EMG) sampling of the abductor pollicis brevis muscle. Neurophysiological studies are not necessarily required pre-operatively if the history and clinical examination fit that of CTS. However, it is considered gold standard for epidemiological studies and it can also be a helpful adjunct in cases where the diagnosis is unclear. Furthermore, it can also be used to provide an objective test and to assess the neurophysiological baseline which can be repeated after surgery for comparison if symptoms are not improving in the long term, e.g., before and after revision carpal tunnel surgery.[7]
Imaging investigations should be considered in cases of diagnostic uncertainty, and both ultrasound and magnetic resonance imaging (MRI) are useful in excluding other causes of median nerve compression within the carpal canal such as mass lesions caused by tumours or cysts. Dynamic ultrasound imaging may demonstrate tenosynovial proliferation and impaired glide.
When diagnostic uncertainty remains, a corticosteroid injection can be used as an adjunct to assess if the patient is given any temporary symptomatic relief. Studies have shown that patients who respond to steroid injection treatment are more likely to have a successful outcome after CTD surgery.[8],[9]
Carpal Tunnel Decompression Surgery
There are two main approaches to CTD, open and endoscopic. The traditional approach is open surgery which produces reliable results with around 90% of patients reporting full resolution or improvement in symptoms.[10],[11] However, open approaches may result in increased scar formation and the palmar surgical scar may be more sensitive in the short term compared to an endoscopic approach.[12] Furthermore, in open CTD, there is a greater risk of nerve tethering in the resulting scar tissue, especially if the incision is placed directly over the nerve. It is therefore important to place the incision ulnar to the median nerve to reduce the risk of this complication. Furthermore, it is essential to continue the carpal tunnel release through the distal forearm fascia ulnar to palmaris longus, to prevent residual fibrotic palmaris insertion points to the palmar aponeurosis compressing the median nerve at the wrist crease.
A mini-open technique for a smaller incision can also be used; however, these endoscopic techniques will have the potential for incomplete decompression due to poorer visualisation. Jugovac et al. compared a 2.5-cm incision with a standard open approach incision from the distal transverse carpal ligament to the proximal wrist crease and found that the mini-open technique resulted in fewer days away from work, better scar cosmesis and no difference in resolution of symptoms.[13]
An endoscopic approach is beneficial in terms of minimal scar, reduced pain and faster return to work.[14],[15] However, one review article showed a higher rate of permanent nerve injury with the endoscopic approach (0.3%) versus the open approach (0.2%).[16]
Evidence demonstrated that at 6-month follow-up, there was no significant difference in symptom relief or functional outcome from either type of surgical approach.[17]
Although many studies show similar long-term results compared to open surgery, as with all endoscopic techniques, there is a learning curve for the surgeons which must be considered with reports of complications, which include incomplete release, transection of the palmar arch and damage to the median nerve as well as digital nerves.[18]
Endoscopic CTD surgery can be a single- or two-port technique and each comes with different challenges.[19],[20] The two-port techniques have had reported cases of ulnar nerve palsy which is more common with a trans-bursal approach as opposed to an extra-bursal.[21] Both techniques are limited in terms of field of vision and can fail to diagnose other carpal tunnel pathology. In a large multicentre study of the single-port technique, the surgeon was not able to visualise the point of blade entry into the transverse carpal ligament in 2.5% of cases, and the majority of these were converted to open procedures.[22]
Although most patients experience a benefit from CTD surgery, a small number reports worsening or no change in their symptoms after surgery.[23],[24] The reasons for failed CTD surgery can be grouped into one of the four categories:
Poor diagnosis
If surgery fails to improve carpal tunnel symptoms, then other diagnoses must be excluded. A possibility is that the nerve problem can be caused by more proximal compression points at the flexor digitorum superficialis, the pronator teres, the lacertus fibrosis and the ligament of Struthers. Concomitant compression of the anterior interosseous nerve can produce forearm pain and weakness and should be excluded when sensory symptoms resolve but the other symptoms persist after CTD. Brachial neuritis, thoracic outlet syndrome and nerve tumours are all associated with sensory disturbance in hand that can mimic median compression neuropathy at the carpal tunnel. Cervical spondylo-radiculopathy is associated with neck pain radiating to corresponding dermatomes with possibly accompanying paraesthesia, weakness and decreased reflexes. C6 root compression produces radial palmar sensory disturbance, but the distribution includes the palmar branch territory, radial forearm and dorsum of the radial hand. This more extensive pattern of sensory impairment should prompt the examiner to consider a proximal root compression or a double crush phenomenon. Examination of the C6 motor innervation (notably biceps, brachialis and brachioradialis) is mandatory to establish whether there is motor involvement from a compressed nerve root.[25] Similar symptoms of weakness, decreased reflexes and sensory loss to affected brachial plexus trunks or cords will manifest in brachial plexus neuritis. Both the previously mentioned conditions can be evaluated using NCSs and EMG.[26] Other diseases such as diabetes causing peripheral neuropathy and mimicking the paraesthesia of CTS, multiple sclerosis, hyperthyroidism or hypothyroidism, rheumatoid arthritis, Lyme disease and other causes of mono-neuropathy are other pathologies which can present with similar symptoms to CTS.[26],[27] Hereditary neuropathy with susceptibility to pressure palsies (HNPP) should be considered when symptoms present in a young patient with a strong previous medical and family history of multiple nerve decompressions. Swelling at the wrist in the region of the carpal tunnel associated with chronic synovitis and tumours or tuberculous infection should be considered. Imaging may be needed to confirm a clinical diagnosis.
Failure to diagnose traction neuritis from a Linburg–Comstock anomaly where an accessory tendon slip or chronic tenosynovial proliferation and encasement between flexor pollicis longus and flexor digitorum profundus of the index finger is present may also cause persistent symptoms.[28] In these cases, the onset of paraesthesia follows activity rather than nocturnal exacerbation.
NCSs and EMG are minimally invasive tests with sensitivity and specificity exceeding 85% and 95%, respectively, and are considered the gold standard for CTS diagnosis.[26],[29],[30] However, any test should always be considered complementary to the clinical findings. In addition to being able to grade the severity of CTS, they also provide a baseline comparison for cases after failed CTD.[31] On the other hand, in primary cases where the diagnosis of CTS is highly unlikely or clinically certain, the results are unlikely to influence the probability of a diagnosis of CTS.[32] Furthermore, NCS may be operator dependent and not look more widely at other pathological causes.
High-resolution ultrasonography has also been used to diagnose CTS based on the median nerve cross-sectional area, with a reported sensitivity and specificity ranging between 57%–89% and 65%–97%, respectively. The higher reported values are for selected patients who already had CTS confirmed on NCS, and the sensitivity and specificity for unselected patients were lower.[26]
Blood tests such glucose, B12 levels, thyroid function and rheumatoid levels are indicated when suspicion of systemic disease is present.[26]
Finally, CTD might not give any functional improvement if there is failure to recognise the functional loss of thumb opposition when the motor branch is already severely injured due to prolonged severe compression with established muscle wasting of the thenar eminence. In this case, an isolated CTD will not restore the function of opposition and other procedures such as tendon transfers are the solution to restore opposition of the thumb and meet patient expectations.
Poor surgery
Surgical error can also lead to failure to achieve a good outcome after surgery. In many cases, this can be attributed to an incomplete decompression. The patient will often report that the symptoms have persisted after surgery and remained unchanged or even deteriorated. The most common location of incomplete release was the distal end of the transverse carpal ligament as shown by Stütz et al. where this was the operative findings in 60% of incomplete carpal tunnel release upon revision surgery.[33] Furthermore, the authors found that the proximal end of the carpal tunnel was incompletely released in 25% of patients in their study and 4.6% of patients had a completely intact transverse carpal ligament.[33] Although early studies have shown a greater incomplete release rate in endoscopic approaches, current studies do not exhibit such discrepancy.[33],[34],[35] It is worthy of note that when a revision decompression is performed, the transverse carpal ligament may be divided in a region slightly different to the original surgery, and to the surgeon, it appears as though the ligament was not released previously as it looks structurally intact. In case of recurrent symptoms where there is an improvement for the first few months with a subsequent return of symptoms, nerve tethering and impaired nerve glide caused by scarring and fibrosis may be the cause. A retrospective study of 200 revision CTDs showed that 23% of patients had a median nerve which was scarred and tethered to the radial wall of the carpal tunnel.[33] This may have been avoided by a more ulnar skin incision.[36],[37]
There is an opportunity to inspect the nerve and its surroundings upon surgical decompression of the carpal tunnel. If the surgeon during the operation fails to diagnose other pathology causing median nerve compression, the symptoms may persist or recur. Missed pathology could include ganglions, synovitis, tumours or accessory muscles and tendons which may be the cause of compression or nerve tether.
Furthermore, iatrogenic injury to the main median nerve or either the palmar or motor branch may cause worsening or new symptoms to appear immediately after surgery. It is therefore essential to be aware of the different anatomical variations of the branches and keep in mind to protect them at all times. Open and endoscopic techniques for CTD in general both have low risk in experienced hands; however, the types of potential iatrogenic injuries differ between the two with the open approach having an extremely low risk of direct injury to the median nerve, ulnar nerve, superficial palmar branch and flexor tendons. The most common nerve injuries following open CTD is injury to the superficial palmar branch followed by common digital nerve injury to the second or third web and motor branch injury.[38],[39] In endoscopic approaches, a study showed that unspecified injury to the median nerve was the most common followed by injury to the common digital nerves, the palmar cutaneous branch and the motor branch. In terms of endoscopic approaches, the third common digital nerve is the most frequently damaged sensory nerve. There was also a much higher incidence of ulnar nerve injury, radial artery injury, ulnar artery injury or injury to the superficial palmar arch.[40] Reports of complete median nerve and ulnar nerve transection have been noted following an endoscopic approach.[41],[42]
Poor nerve
Surgical decompression of the carpal tunnel may not improve symptoms if there is already a 'poor nerve'. This is when the timing of decompression follows an already prolonged duration of severe nerve compression and established axonopathy with loss of axons rendering the injury permanent. The pathophysiology of CTS follows compression of the median nerve which is caused by an increased carpal tunnel pressure. The carpal tunnel pressure can be increased in patients with CTS when the wrist is positioned in either extension or flexion which is the bases for clinical tests such as Phalen's and reverse Phalen's test. Furthermore, the carpal tunnel pressure can be abnormally high even in a neutral position for some patients.[43] This can cause disruption to the blood–nerve barrier with ensuing endoneurial oedema, inflammation, perineural thickening and fibrosis of surrounding tissues caused by increased fibroblastic activity around the nerve.[44],[45],[46],[47] Intermittent nerve ischaemia and reperfusion in periods of recovery can lead to oxidative stress by the release of free radicals. As myelinated nerves have a higher proportion of lipids acting as target for oxidative free radicals, these nerves are more severely affected compared to non-myelinated nerve fibres.[47],[48] Subsequently, this leads to ischaemia of the microneurial environment and further results in demyelination and lastly axonal death.[49] In these cases, and cases with concomitant diabetic neuropathy, it is essential to manage the patients' expectations from the outset regarding what outcomes to realistically expect.
Poor luck
Unsatisfying CTD outcomes can arise from complications of the surgery itself. These complications cause pain and reduced function.
Pillar pain and scar sensitivity are the two most common complications that patients encounter. There is no consensus on the definition of pillar pain, but it is commonly described as pain in the thenar or hypothenar areas. Studies show a post-operative incidence rate of 19%–36%, and for most patients, this resolves within 3 months from surgery.[50] Scar tenderness is another troublesome complication that usually fully resolves; however, Kharwadkar et al. found that in 50%–80% of patients, this was still present at 3 months post-operatively.[51] In addition, as with any surgical hand wound, there is the risk of surgical site infection, but fortunately, this is rare at an incidence between 0.03% and 0.47%.[52],[53]
Complex regional pain syndrome (CRPS) although rare is a debilitating complication of surgical decompression where patients present with a stiff, swollen and painful hand. The Budapest diagnostic criteria for CRPS include features such as pain out of proportion to any triggering stimulus, vasomotor, sensory, pseudomotor and trophic changes. CRPS is not exhibited in a specific dermatome or nerve distribution. There are two types of CRPS with type I diagnosed according to the criteria above and type II if the condition also includes definite evidence of a major nerve lesion. Assessment for a nerve lesion should be thorough and neurophysiology may be able to demonstrate whether there has been any deterioration or function loss following surgery.[54] In such cases, the nerve may need to be re-explored with an indwelling nerve catheter for a few days to break the pain cycle. The patient cohort at risk of CRPS from primary CTD includes genetic factors, a history of migraine, Angiotensin-converting enzyme (ACE)inhibitor medication, female gender and those with a need for immobilisation of an injured limb.[55]
Fortunately, there are ways to treat the symptoms including pain medications, neuromodulation, psychological support, mirror therapy, exposure therapy, physiotherapy, hand therapy and desensitisation; however, recovery may take years.[54]
Patients with peripheral neuropathy and a nerve entrapment (including diabetic patients) have good outcomes following CTD with 92% improvement and 72% complete resolution in one study; hence, this is not a contraindication to surgery.[56] Mondelli et al. showed that a case-matched study of diabetic and non-diabetic patients did not have any difference in outcome following CTD.[57] Patients should be informed that the sensory and motor loss may not recover if there is significant neuropathy; however, paraesthesia implies a reversible nerve dysfunction and this is usually improved. Rarely, patients with significant neuropathy have increased pain after decompression, perhaps thought to be related to reperfusion in a previously ischaemic nerve.
Approach to the Patient With Failed Carpal Tunnel Surgery
It is vital to apply a systematic approach to patients who have no symptom relief, worsening of symptoms or new symptoms after CTD surgery. The aim is to find out the underlying cause for the failed surgery as grouped by the four categories previously mentioned.
Revisiting the history
Establishing the original onset of the symptoms and response to the surgery is crucial. If new symptoms appear after surgery, this leads one to suspect iatrogenic nerve injury; whereas if a period of complete resolution is followed by recurrence of symptoms, this suggests true recurrence due to contraction at the site of healing of the transverse carpal ligament release and perhaps scar tether involving the median nerve. It is also important to ask about exacerbating activities and occupational provocations and changes in daily activities and work as well as previous of new-onset symptoms of neck pain. Psychological and social factors including occupational disputes, personal injury, depression, anxiety, chronic pain syndromes, social isolation and medicolegal claims are associated with impaired resilience and poor compliance. Whenever possible, these issues should be explored in cases of failed CTD surgery. These factors will otherwise continue to influence the outcome of any subsequent intervention.
A comprehensive review of comorbidities and family history is also warranted as familial conditions can predispose to CTS such as hereditary neuropathy with sensitivity to pressure palsies or mimic hand dysfunction such as Charcot-Marie-tooth disease, a hereditary sensory-motor distal neuropathy. Studies have shown that this patient cohort who undergo carpal tunnel release does experience improvement in symptoms after surgical release and Panosyan et al. found a 93% satisfaction rate in their study.[58] Patients with hereditary neuropathy and liability to pressure palsies have either a deletion or abnormality of the peripheral myelin protein 22 gene, which results in an unstable myelin sheath liable to injury during minor stretch or compression. Electrophysiological studies show conduction block or decreased velocity through the median nerve. However, the pathophysiology lies within the stability of the myelin sheath rather than new myelin formation and conduction repair, and cases of patients with complete resolution of carpal tunnel symptoms and normalisation of electrophysiological tests after decompression have been reported.[59]
Revisiting the examination
The examination should comprise the entire neural pathway from the central nervous system to the hand. In hand, one should assess Tinel's, Phalen's and direct compression tests. The scratch-collapse test may be useful in demonstrating a site of residual compression and pain. The function of the main median nerve, motor branch and palmar cutaneous branch sensation should be carefully assessed and recorded. The position of the surgical scar may provide a clue as to the reasons for a poor outcome. Sensitivity in the scar may imply a cutaneous neuroma, and this can be assessed further with local anaesthetic injection superficially at the site of pain, with a complete resolution being suggestive of a palmar cutaneous branch injury. One should then continue to assess for proximal signs of compression including the pronator syndrome. Furthermore, a brachial plexus examination should be undertaken to exclude brachial plexopathy or compression at the thoracic outlet. In addition, symptoms can arise from compression at the cervical spinal nerve root level in cervical radiculopathy or cervical myelopathy, resulting in symptoms similar to CTS.[26] The cranial nerves and the lower limbs should also be tested to exclude any other neurological pathology. Rarely, multiple sclerosis is diagnosed in the clinic when patients present with persistent numbness or paraesthesia after CTD.
In rare cases, median nerve symptoms can be caused by traction neuritis, as in the case of a Linburg–Comstock anomaly consisting of an extra tendon slip between flexor pollicis longus and flexor digitorum profundus to the index finger or an associated chronic tenosynovium encasement tethering the median nerve to the flexor tendons. Steroid injection may help alleviate symptoms, and so, patients will often present after failed CTD. The distal interphalangeal joint of the index flexes as the thumb is flexed and this should prompt further evaluation as a potential cause of residual symptoms. The clinical test for this condition is passive stretch of the index finger to extension with the thumb flexed fully at the interphalangeal joint across the palm. A positive test is elicited with sharp nerve pain and paraesthesia in the median distribution which tracks from the distal radial volar forearm to the fingertips and is improved after release of the index finger.
Investigations
Neurophysiology assessment is essential for patients after failed carpal tunnel surgery to look for changes from the baseline results if these were performed previously. A more extensive assessment includes evaluation for proximal pathology, spinal root involvement and other peripheral nerves entrapments. Lower limbs assessment is useful in neuropathy and myelopathy and to look for other entrapment sites in HNPP. There are uncommon interconnections between the ulnar nerve and median nerve in the forearm, the carpal tunnel and the hand at the common digital nerve level that may result in diagnostic confusion and a false-positive diagnosis of carpal tunnel with a proximal ulnar nerve compression. As a general rule, if repeat neurophysiological assessment demonstrates improvement in the conduction parameters of the median nerve, it is reasonable to clinically monitor for a few months anticipating further improvement. If symptoms are unchanged, worse or new pathology is identified, and revision nerve exploration is warranted.
In isolated cases, a dynamic ultrasound can help assess nerve glide within the carpal tunnel. Furthermore, if indicated, an MRI of the cervical spine to delineate root pathology or an MRI of the carpal tunnel to assess for other pathology and synovitis may be helpful. Blood tests may need to be undertaken to screen for systemic conditions which are associated with or produce symptoms similar to CTS. These include glucose for diabetes, thyroid function tests for hypothyroidism, B12 and folate for peripheral neuropathy and rheumatoid screening for rheumatoid arthritis.[26],[60]
Reviewing previous records
Previous medical records can be very helpful in identifying the cause of a failed CTD. Reviewing the recorded medical history and neurophysiology results will give a good baseline impression. It is essential to review the operation notes with regards to the documented procedure and findings, the surgeon grade and experience, the anaesthetic records and tourniquet time. Hand therapy follow-up and progress are also helpful to establish the full picture of the patient condition, including patient understanding and expectations and to establish compliance with rehabilitation and future treatment goals.
- Carpal tunnel release review at 6 weeks
- No relief of symptoms/worsening symptoms
- Review operations, notes and pre-operative assessment. In case of severe CTS, symptoms temporarily worsen during recovery. May not get complete recovery [Diagram 1].
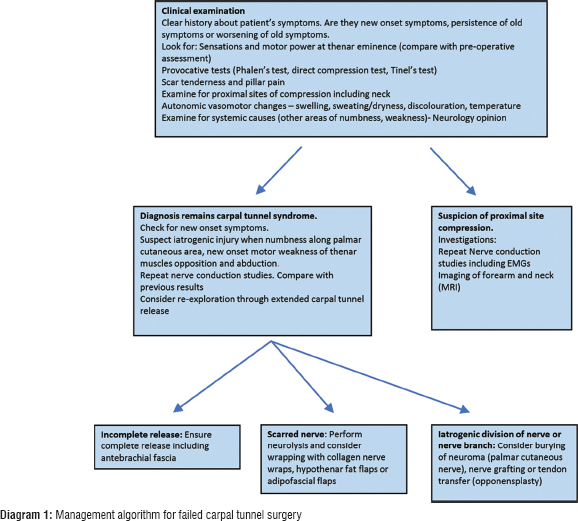
Revision surgery
Worse, persistent or new symptoms should be considered indications for revision surgery. Studies have shown that the most common reason for failed primary decompression surgery is incomplete release of the transverse carpal ligament.[33] Although possible to perform under a local anaesthetic or using a wide-awake local anaesthesia no tourniquet technique, the lead author prefers an axillary regional anaesthetic block and arm tourniquet to allow sufficient time for exploration, neurolysis and managing any unexpected findings at the time of revision surgery. A tourniquet assists in maintaining a bloodless field during neurolysis. Loupe magnification should be used.
At revision surgery, an extended carpal tunnel incision crossing the wrist crease with angulation to the ulnar side of the forearm is used to fully evaluate the distal forearm fascia and the palmaris longus insertion when present.
A revision release of the transverse carpal ligament is performed with full release confirmed through visual identification and with external neurolysis of the median nerve in every case. On rare occasions, an internal neurolysis may be indicated by a tight constriction of the epineurium. In these rare cases, a longitudinal epineurotomy and limited internal neurolysis are performed with magnification from an operating microscope.
The motor branch should be identified and its course to the thenar muscle should be assessed and documented. If constricted within or at the distal edge of the flexor retinaculum, the motor branch should be formally release.
In cases of a detected nerve injury, nerve grafting should be performed after neuroma excision under the operating microscope. Autologous nerve graft from the ipsilateral lateral cutaneous nerve of the forearm or from the medial cutaneous nerve of the arm are the obvious donor choice to avoid dual limb operating for a sural nerve harvest which may require general anaesthesia. In cases with extreme neuropathic pain, nerve sensitivity at the site of injury and central sensitisation, the lead author prefers to use AVANCE® processed nerve allograft (AxoGen Inc. Calchua, FL, USA) for bridging the nerve gap, rather than risking a second site of nerve sensitivity and the morbidity from numbness at an autologous nerve graft harvest site[61] [Figure - 1]a and [Figure - 1]b.
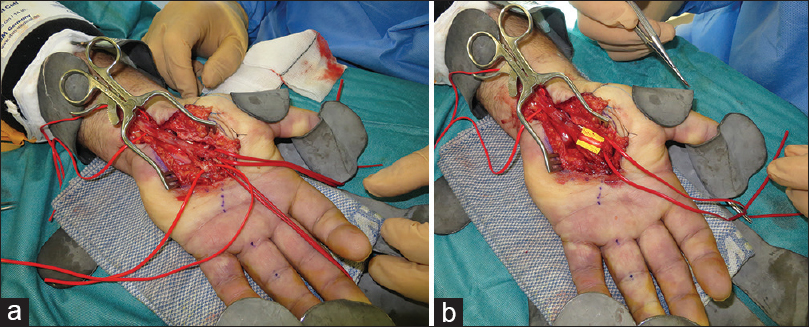 |
| Figure 1: (a) Iatropathic injury to the first web space nerves identified at revision carpal tunnel decompression, (b) AVANCE™ processed nerve allograft reconstruction |
In cases of re-revision or first revision with extensive scar tether of the median nerve or epineurium damage, a resurfacing procedure may be indicated. Autologous biological vascularised tissues can be harvested and interposed between the median nerve and tendons or skin. The radial artery perforator adipo-fascial flap may be dissected and rotated distally to cover the nerve as it passes from the distal forearm to the carpal tunnel. Care should be taken when raising the flap to avoid injury to the terminal branches of the lateral cutaneous nerve of the forearm. The flap may be taken with skin to resurface the area of the carpal tunnel in cases of extensive scar from trauma, infection or repeated surgery.
Tham et al. published on the outcome of the reverse radial forearm flap for revision carpal tunnel surgery in which six patients experienced improvement in symptoms of which two had complete resolution of pain.[62] Mahmoud reported six patients who underwent the same procedure and was followed up for an average of 2 years with no worsening in symptoms and all patients reporting disappearance of night time symptoms. Three of those still experienced persistent paraesthesia.[63]
The ulnar artery perforator adipo-fascial flap has also been described as an option for median nerve soft tissue coverage with several small studies published all with good outcomes of either improvement or full resolution of symptoms.[64],[65],[66] This flap which may be elevated with skin as a Becker flap from the ulnar artery can be used in a similar way [Figure - 2].
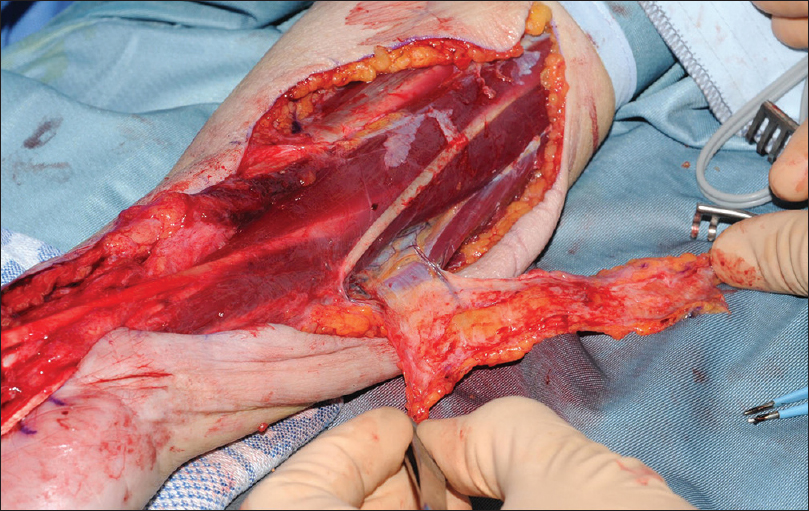 |
| Figure 2: Ulnar adipofascial perforator flap |
In cases where there is sufficient skin cover but early reformation of the flexor retinaculum with scar tether to the median nerve, a turnover vascularised hypothenar fat pan may be interposed between the freshly cut edges of the retinaculum at the time of revision surgery. This tissue acts as a barrier to early healing of the retinaculum and provides cushioning or a sensitive nerve plus a gliding layer to reduce the risk of recurrent adhesions to the median nerve.
There has been several case series published on the hypothenar fat pad flap with the majority reporting good outcomes following surgery. Fusetti et al. found a 90% resolution of pain by 6 months following decompression, and Mathoulin et al. and Craft et al. had improvement or complete disappearance of pain and numbness in 94% and 83% of patients, respectively.[67],[68],[69] Athlani and Haloua reported improvement in pain and paraesthesia along with improved QuickDASH score and objective grip strength in a series of 34 patients followed up at 2 years who underwent revision carpal tunnel surgery with hypothenar fat pad coverage.[70] Similarly, Wichelhaus et al. reported symptomatic improvement and satisfaction of the procedure in 83% out of 18 patients who had revision carpal tunnel surgery with a hypothenar flap. The remaining three patients however did not experience any improvement in this series.[71] Pace et al. compared self-reported patient outcomes using the Boston Carpal Tunnel Questionnaire in 17 patients who underwent repeat CTD alone versus 16 patients who had repeat decompression and a hypothenar fat flap. Their study found no statistical difference between the groups (P = 0.09).[72]
Two studies describe use of a free non-vascularised vein graft split longitudinally and then sutured around the median nerve in a spiral configuration as a nerve wrap. All patients demonstrated improved symptoms, two-point discrimination and electrophysiological testing. However, the patient numbers were small with only 3 and 19 included, respectively.[73],[74]
In cases where patients have had multiple revision carpal tunnel surgeries and failed local flap options, the literature describes free flaps as alternatives for median nerve cover. A case series by Goitz and Steichen on nine patients undergoing free omental flap coverage followed up for at least 4 years with a majority experiencing improvement in some symptoms, but none had any complete resolution. In addition, none of the patients managed to get back to work at final follow-up.[75]
Commercially available nerve wrapping materials may be used to prevent scar formation onto the median nerve at revision surgery. The AxoGuard® nerve protector (AxoGen Inc. Calchua, FL, USA) is a layered collagen extracellular matrix membrane processed from the submucosa of porcine small intestine.[76] It is supplied in a number of sizes and after soaking in saline is sutured loosely around the nerve providing a layer that re-vascularises and restores gliding without surgical bed scar reforming to the epineurium [Figure - 3]a and [Figure - 3]b. The Vivosorb™ (Polyganics, Netherlands) is a polymer membrane of polycaprolactone that provides a temporary barrier to scar.[77] The polymer undergoes hydrolysis and forms a hydrogel layer that eventually resorbs by approximately 18 months. NeuraWrap™ nerve protector (Integra Life Sciences) is an absorbable collagen membrane that can be placed around a damaged nerve to prevent scar adhesion.[78] The benefit of these products is that they can be readily available and have no donor morbidity associated with their use.[79] There is limited clinical evidence available in revision nerve surgery to date.[80]
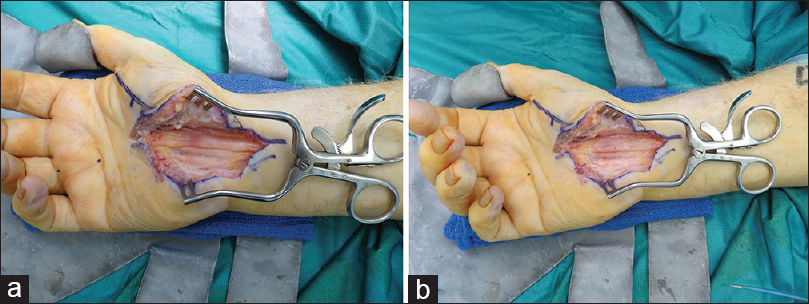 |
| Figure 3: (a) Revision carpal tunnel decompression at three months for worsening symptoms – incomplete decompression on revision exploration, (b) AxoGuard™ nerve protector wrap |
For revision cases with complete loss of thenar muscle function, restoration of thumb palmar abduction and opposition with an opponensplasty tendon transfer should be considered using either the extensor indicis proprius or the flexor digitorum superficialis to the ring finger, redirected through a distally based flexor carpi ulnaris pulley.
In cases of revision CTD with pre-operative neuropathic pain, surgical placement of an indwelling local anaesthetic nerve block catheter for 48 h is a useful consideration.
Meticulous haemostasis before closure reduces the risk of a post-operative haematoma. Interrupted non-absorbable sutures are used for skin closure and a bulky dressing applied. A volar plaster slab can be used with 15° of wrist extension to support the wrist until the first dressing change.
Prophylactic antibiotics are administered when a non-vascularised barrier membrane is inserted.
Revision carpal tunnel surgery is less predictable than primary CTD. Jones et al. reported 75% of patients with improvement of symptoms and 50% of patients having complete resolution of pain. Steyers reported that many patients still have some persistent symptoms after a revision decompression at the carpal tunnel with rates between 41% and 90%.[1],[81]
Conclusion
Revision CTD surgery makes up 2.7% of all carpal tunnel surgery. Causes of deterioration, no change, partial improvement or recurrent symptoms may be due to poor diagnosis, poor surgery, poor nerve or poor luck. We outlined a systematic approach and an algorithm for revision surgery in the failed carpal tunnel surgery patient. Revision surgery in the majority of patients results in good outcomes with improvement in symptoms.
Ethical approval
None required. All patients have consented to having their intraoperative photographs used for publication purposes.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Author's contributions
MV, RMJ and DP conceived the study and edited all the drafts. MV performed the literature search, analysed and summarised the data and wrote the initial draft. RMJ and DP provided senior support, clinical photographs and advice. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Jones NF, Ahn HC, Eo S. Revision surgery for persistent and recurrent carpal tunnel syndrome and for failed carpal tunnel release. Plast Reconstr Surg 2012;129:683-92. [Google Scholar] |
| 2. | Aroori S, Spence RA. Carpal tunnel syndrome. Ulster Med J 2008;77:6-17. [Google Scholar] |
| 3. | Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry 2006;77:263-5. [Google Scholar] |
| 4. | NHS England HES. Hospital Admitted Patient Care Activity, 2017-18: Procedures and Interventions; 2017-18. Available from: https://www.digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2017-18. [Last accessed 2018 Nov 20]. [Google Scholar] |
| 5. | Massy-Westropp N, Grimmer K, Bain G. A systematic review of the clinical diagnostic tests for carpal tunnel syndrome. J Hand Surg Am 2000;25:120-7. [Google Scholar] |
| 6. | MacDermid JC, Wessel J. Clinical diagnosis of carpal tunnel syndrome: A systematic review. J Hand Ther 2004;17:309-19. [Google Scholar] |
| 7. | Werner RA, Andary M. Carpal tunnel syndrome: Pathophysiology and clinical neurophysiology. Clin Neurophysiol 2002;113:1373-81. [Google Scholar] |
| 8. | Edgell SE, McCabe SJ, Breidenbach WC, LaJoie AS, Abell TD. Predicting the outcome of carpal tunnel release. J Hand Surg Am 2003;28:255-61. [Google Scholar] |
| 9. | Green DP. Diagnostic and therapeutic value of carpal tunnel injection. J Hand Surg Am 1984;9:850-4. [Google Scholar] |
| 10. | Huisstede BM, Randsdorp MS, Coert JH, Glerum S, van Middelkoop M, Koes BW, et al. Carpal tunnel syndrome. Part II: Effectiveness of surgical treatments – A systematic review. Arch Phys Med Rehabil 2010;91:1005-24. [Google Scholar] |
| 11. | Gerritsen AA, de Vet HC, Scholten RJ, Bertelsmann FW, de Krom MC, Bouter LM, et al. Splinting vs surgery in the treatment of carpal tunnel syndrome: A randomized controlled trial. JAMA 2002;288:1245-51. [Google Scholar] |
| 12. | Thoma A, Veltri K, Haines T, Duku E. A meta-analysis of randomized controlled trials comparing endoscopic and open carpal tunnel decompression. Plast Reconstr Surg 2004;114:1137-46. [Google Scholar] |
| 13. | Jugovac I, Burgić N, Mićović V, Radolović-Prenc L, Uravić M, Golubović V, et al. Carpal tunnel release by limited palmar incision vs. traditional open technique: Randomized controlled trial. Croat Med J 2002;43:33-6. [Google Scholar] |
| 14. | Atroshi I, Larsson GU, Ornstein E, Hofer M, Johnsson R, Ranstam J, et al. Outcomes of endoscopic surgery compared with open surgery for carpal tunnel syndrome among employed patients: Randomised controlled trial. BMJ 2006;332:1473. [Google Scholar] |
| 15. | Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release: A prospective, randomized trial. J Bone Joint Surg Am 2002;84-A: 1107-15. [Google Scholar] |
| 16. | Boeckstyns ME, Sørensen AI. Does endoscopic carpal tunnel release have a higher rate of complications than open carpal tunnel release? An analysis of published series. J Hand Surg Br 1999;24:9-15. [Google Scholar] |
| 17. | Sayegh ET, Strauch RJ. Open versus endoscopic carpal tunnel release: A meta-analysis of randomized controlled trials. Clin Orthop Relat Res 2015;473:1120-32. [Google Scholar] |
| 18. | Kelly CP, Pulisetti D, Jamieson AM. Early experience with endoscopic carpal tunnel release. J Hand Surg Br 1994;19:18-21. [Google Scholar] |
| 19. | Agee JM, McCarroll HR, North ER. Endoscopic carpal tunnel release using the single proximal incision technique. Hand Clin 1994;10:647-59. [Google Scholar] |
| 20. | Chow JC. Endoscopic release of the carpal ligament: A new technique for carpal tunnel syndrome. Arthroscopy 1989;5:19-24. [Google Scholar] |
| 21. | Chow JC, Hantes ME. Endoscopic carpal tunnel release: Thirteen years' experience with the chow technique. J Hand Surg Am 2002;27:1011-8. [Google Scholar] |
| 22. | Agee JM, Peimer CA, Pyrek JD, Walsh WE. Endoscopic carpal tunnel release: A prospective study of complications and surgical experience. J Hand Surg Am 1995;20:165-71. [Google Scholar] |
| 23. | Jarvik JG, Comstock BA, Kliot M, Turner JA, Chan L, Heagerty PJ, et al. Surgery versus non-surgical therapy for carpal tunnel syndrome: A randomised parallel-group trial. Lancet 2009;374:1074-81. [Google Scholar] |
| 24. | Cseuz KA, Thomas JE, Lambert EH, Love JG, Lipscomb PR. Long-term results of operation for carpal tunnel syndrome. J Occup Environ Med 1966;8:560. [Google Scholar] |
| 25. | Kwon HK, Hwang M, Yoon DW. Frequency and severity of carpal tunnel syndrome according to level of cervical radiculopathy: Double crush syndrome? Clin Neurophysiol 2006;117:1256-9. [Google Scholar] |
| 26. | Kleopa KA. In the clinic. Carpal tunnel syndrome. Ann Intern Med 2015;163:ITC1. [Google Scholar] |
| 27. | Shores JT, Lee WP. An evidence-based approach to carpal tunnel syndrome. Plast Reconstr Surg 2010;126:2196-204. [Google Scholar] |
| 28. | Slater RR. Flexor tendon anomalies in a patient with carpal tunnel syndrome. J Hand Surg Br 2001;26:373-6. [Google Scholar] |
| 29. | Jablecki CK, Andary MT, So YT, Wilkins DE, Williams FH. Literature review of the usefulness of nerve conduction studies and electromyography for the evaluation of patients with carpal tunnel syndrome. AAEM quality assurance committee. Muscle Nerve 1993;16:1392-414. [Google Scholar] |
| 30. | Lo JK, Finestone HM, Gilbert K, Woodbury MG. Community-based referrals for electrodiagnostic studies in patients with possible carpal tunnel syndrome: What is the diagnosis? Arch Phys Med Rehabil 2002;83:598-603. [Google Scholar] |
| 31. | Bland JD. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve 2000;23:1280-3. [Google Scholar] |
| 32. | Graham B. The value added by electrodiagnostic testing in the diagnosis of carpal tunnel syndrome. J Bone Joint Surg Am 2008;90:2587-93. [Google Scholar] |
| 33. | Stütz N, Gohritz A, van Schoonhoven J, Lanz U. Revision surgery after carpal tunnel release – Analysis of the pathology in 200 cases during a 2 year period. J Hand Surg Br 2006;31:68-71. [Google Scholar] |
| 34. | Forman DL, Watson HK, Caulfield KA, Shenko J, Caputo AE, Ashmead D, et al. Persistent or recurrent carpal tunnel syndrome following prior endoscopic carpal tunnel release. J Hand Surg Am 1998;23:1010-4. [Google Scholar] |
| 35. | Benson LS, Bare AA, Nagle DJ, Harder VS, Williams CS, Visotsky JL, et al. Complications of endoscopic and open carpal tunnel release. Arthroscopy 2006;22:919-24, 924.e1-2. [Google Scholar] |
| 36. | Giunta R, Frank U, Lanz U. The hypothenar fat-pad flap for reconstructive repair after scarring of the median nerve at the wrist joint. Chir Main 1998;17:107-12. [Google Scholar] |
| 37. | Wulle C. Treatment of recurrence of the carpal tunnel syndrome. Ann Chir Main 1987;6:203-9. [Google Scholar] |
| 38. | Einhorn N, Leddy JP. Pitfalls of endoscopic carpal tunnel release. Orthop Clin North Am 1996;27:373-80. [Google Scholar] |
| 39. | MacDonald RI, Lichtman DM, Hanlon JJ, Wilson JN. Complications of surgical release for carpal tunnel syndrome. J Hand Surg Am 1978;3:70-6. [Google Scholar] |
| 40. | Palmer AK, Toivonen DA. Complications of endoscopic and open carpal tunnel release. J Hand Surg Am 1999;24:561-5. [Google Scholar] |
| 41. | Malek M, Chow J, Veech D, editors. Complications of Endoscopic carpal tunnel release: analysis of 10624 cases. Annual Meeting of he American Association of Orthopaedic Surgeons. New Orleans; 1994. [Google Scholar] |
| 42. | Resnick CT, Miller BW. Endoscopic carpal tunnel release using the subligamentous two-portal technique. Contemp Orthop 1991;22:269-77. [Google Scholar] |
| 43. | Seradge H, Jia YC, Owens W.In vivo measurement of carpal tunnel pressure in the functioning hand. J Hand Surg Am 1995;20:855-9. [Google Scholar] |
| 44. | Lundborg G, Dahlin LB. Anatomy, function, and pathophysiology of peripheral nerves and nerve compression. Hand Clin 1996;12:185-93. [Google Scholar] |
| 45. | Lundborg G, Myers R, Powell H. Nerve compression injury and increased endoneurial fluid pressure: A “miniature compartment syndrome”. J Neurol Neurosurg Psychiatry 1983;46:1119-24. [Google Scholar] |
| 46. | Mackinnon SE. Pathophysiology of nerve compression. Hand Clin 2002;18:231-41. [Google Scholar] |
| 47. | Mackinnon SE, Dellon AL, Hudson AR, Hunter DA. Chronic nerve compression – An experimental model in the rat. Ann Plast Surg 1984;13:112-20. [Google Scholar] |
| 48. | O'Brien JP, Mackinnon SE, MacLean AR, Hudson AR, Dellon AL, Hunter DA, et al. Amodel of chronic nerve compression in the rat. Ann Plast Surg 1987;19:430-5. [Google Scholar] |
| 49. | Pritsch T, Rosenblatt Y, Carmel A. Carpal tunnel syndrome. Harefuah 2004;143:743-8, 765, 764. [Google Scholar] |
| 50. | Ludlow KS, Merla JL, Cox JA, Hurst LN. Pillar pain as a postoperative complication of carpal tunnel release: A review of the literature. J Hand Ther 1997;10:277-82. [Google Scholar] |
| 51. | Kharwadkar N, Naique S, Molitor PJ. Prospective randomized trial comparing absorbable and non-absorbable sutures in open carpal tunnel release. J Hand Surg Br 2005;30:92-5. [Google Scholar] |
| 52. | Hanssen AD, Amadio PC, DeSilva SP, Ilstrup DM. Deep postoperative wound infection after carpal tunnel release. J Hand Surg Am 1989;14:869-73. [Google Scholar] |
| 53. | Harness NG, Inacio MC, Pfeil FF, Paxton LW. Rate of infection after carpal tunnel release surgery and effect of antibiotic prophylaxis. J Hand Surg Am 2010;35:189-96. [Google Scholar] |
| 54. | Harden RN, Oaklander AL, Burton AW, Perez RS, Richardson K, Swan M, et al. Complex regional pain syndrome: Practical diagnostic and treatment guidelines, 4th edition. Pain Med 2013;14:180-229. [Google Scholar] |
| 55. | Marinus J, Moseley GL, Birklein F, Baron R, Maihöfner C, Kingery WS, et al. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol 2011;10:637-48. [Google Scholar] |
| 56. | Clayburgh RH, Beckenbaugh RD, Dobyns JH. Carpal tunnel release in patients with diffuse peripheral neuropathy. J Hand Surg Am 1987;12:380-3. [Google Scholar] |
| 57. | Mondelli M, Padua L, Reale F, Signorini AM, Romano C. Outcome of surgical release among diabetics with carpal tunnel syndrome. Arch Phys Med Rehabil 2004;85:7-13. [Google Scholar] |
| 58. | Panosyan FB, Kirk CA, Marking D, Reilly MM, Scherer SS, Shy ME, et al. Carpal tunnel syndrome in inherited neuropathies: A retrospective survey. Muscle Nerve 2018;57:388-94. [Google Scholar] |
| 59. | Earle N, Zochodne DW. Is carpal tunnel decompression warranted for HNPP? J Peripher Nerv Syst 2013;18:331-5. [Google Scholar] |
| 60. | Stevens JC, Beard CM, O'Fallon WM, Kurland LT. Conditions associated with carpal tunnel syndrome. Mayo Clin Proc 1992;67:541-8. [Google Scholar] |
| 61. | Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, et al. Processed nerve allografts for peripheral nerve reconstruction: A multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery 2012;32:1-4. [Google Scholar] |
| 62. | Tham SK, Ireland DC, Riccio M, Morrison WA. Reverse radial artery fascial flap: A treatment for the chronically scarred median nerve in recurrent carpal tunnel syndrome. J Hand Surg Am 1996;21:849-54. [Google Scholar] |
| 63. | Mahmoud M. The use of perforator radial fascial forearm flap in the management of recurrent carpal tunnel syndrome. Egypt Orthop J 2015;50:96. [Google Scholar] |
| 64. | Adani R, Tos P, Tarallo L, Corain M. Treatment of painful median nerve neuromas with radial and ulnar artery perforator adipofascial flaps. J Hand Surg Am 2014;39:721-7. [Google Scholar] |
| 65. | McInerney NM, Hussey AJ. The ulnar artery perforator adipofascial flap: An alternative for vascularised coverage of the median nerve. J Plast Reconstr Aesthet Surg 2017;70:547-9. [Google Scholar] |
| 66. | Noor S, Rajaratnam V, Jose R. The adipofascial flap based on an ulnar artery perforator: An alternative option for recurrent carpal tunnel syndrome. J Hand Surg Eur Vol 2012;37:895. [Google Scholar] |
| 67. | Craft RO, Duncan SF, Smith AA. Management of recurrent carpal tunnel syndrome with microneurolysis and the hypothenar fat pad flap. Hand (N Y) 2007;2:85-9. [Google Scholar] |
| 68. | Fusetti C, Garavaglia G, Mathoulin C, Petri JG, Lucchina S. A reliable and simple solution for recalcitrant carpal tunnel syndrome: The hypothenar fat pad flap. Am J Orthop (Belle Mead NJ) 2009;38:181-6. [Google Scholar] |
| 69. | Mathoulin C, Bahm J, Roukoz S. Pedicled hypothenar fat flap for median nerve coverage in recalcitrant carpal tunnel syndrome. Hand Surg 2000;5:33-40. [Google Scholar] |
| 70. | Athlani L, Haloua JP. Strickland's hypothenar fat pad flap for revision surgery in carpal tunnel syndrome: Prospective study of 34 cases. Hand Surg Rehabil 2017;36:202-7. [Google Scholar] |
| 71. | Wichelhaus A, Mittlmeier T, Gierer P, Beck M. Vascularized hypothenar fat pad flap in revision surgery for carpal tunnel syndrome. J Neurol Surg A Cent Eur Neurosurg 2015;76:438-42. [Google Scholar] |
| 72. | Pace GI, Zale CL, Gendelberg D, Taylor KF. Self-reported outcomes for patients undergoing revision carpal tunnel surgery with or without hypothenar fat pad transposition. Hand (N Y) 2018;13:292-5. [Google Scholar] |
| 73. | Sotereanos DG, Giannakopoulos PN, Mitsionis GI, Xu J, Herndon JH. Vein-graft wrapping for the treatment of recurrent compression of the median nerve. Microsurgery 1995;16:752-6. [Google Scholar] |
| 74. | Varitimidis SE, Vardakas DG, Goebel F, Sotereanos DG. Treatment of recurrent compressive neuropathy of peripheral nerves in the upper extremity with an autologous vein insulator. J Hand Surg Am 2001;26:296-302. [Google Scholar] |
| 75. | Goitz RJ, Steichen JB. Microvascular omental transfer for the treatment of severe recurrent median neuritis of the wrist: A long-term follow-up. Plast Reconstr Surg 2005;115:163-71. [Google Scholar] |
| 76. | Papatheodorou LK, Williams BG, Sotereanos DG. Preliminary results of recurrent cubital tunnel syndrome treated with neurolysis and porcine extracellular matrix nerve wrap. J Hand Surg Am 2015;40:987-92. [Google Scholar] |
| 77. | Siemers F, Houschyar K. Alternative Strategies for Nerve Reconstruction. Modern Concepts of Peripheral Nerve Repair. Cham, Switzerland: Springer; 2017. p. 79-96. [Google Scholar] |
| 78. | Soltani AM, Allan BJ, Best MJ, Mir HS, Panthaki ZJ. Revision decompression and collagen nerve wrap for recurrent and persistent compression neuropathies of the upper extremity. Ann Plast Surg 2014;72:572-8. [Google Scholar] |
| 79. | Shintani K, Uemura T, Takamatsu K, Yokoi T, Onode E, Okada M, et al. Protective effect of biodegradable nerve conduit against peripheral nerve adhesion after neurolysis. J Neurosurg 2018;129:815-24. [Google Scholar] |
| 80. | Abzug JM, Jacoby SM, Osterman AL. Surgical options for recalcitrant carpal tunnel syndrome with perineural fibrosis. Hand (N Y) 2012;7:23-9. [Google Scholar] |
| 81. | Steyers CM. Recurrent carpal tunnel syndrome. Hand Clin 2002;18:339-45. [Google Scholar] |
Fulltext Views
21,970
PDF downloads
1,189





