Translate this page into:
Iatrogenic nerve injuries in orthopaedics
2 Burns and Plastics, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK
3 West Midlands Brachial Plexus and Peripheral Nerve Injury Service, University Hospitals Birmingham NHS Foundation Trust; Honorary Senior Clinical Lecturer, University of Birmingham, Birmingham, UK
Corresponding Author:
Jun Yi Soh
Queen Elizabeth University Hospitals Birmingham NHS Foundation Trust, Mindelsohn Way, Birmingham B15 2TH, Birmingham
UK
junyi.soh@nhs.net
| How to cite this article: Soh J, Hill J, Power DM. Iatrogenic nerve injuries in orthopaedics. J Musculoskelet Surg Res 2019;3:9-14 |
Abstract
Peripheral nerves are at risk of injury during both orthopaedic trauma and elective surgery. The aims of this review are to raise awareness of iatrogenic nerve injuries and recommend strategies clinicians can employ to aid diagnosis, management and improve recovery for patients. A thorough understanding of anatomy and appreciation of pathoanatomy in fracture surgery can help reduce this risk. When injuries occur, a systematic clinical assessment can usually locate the site of injury, and with a repeated examination, the physiological grade can be determined. The neurapraxic injury may deteriorate if the conditions around the nerve are unfavourable. Caution should be exerted when making a diagnosis of conduction block because close, regular clinical monitoring is necessary to detect any deterioration early. Surgery may be required in such injuries if there is a failure to progress or deterioration occurs. Higher grade injuries may require surgical exploration and reconstruction. These procedures are most appropriately performed by specialist surgeons working in regional nerve injury units. Early diagnosis and management of an iatrogenic injury reduces the emotional impact, may prevent central pain sensitisation and ensures the optimum chance of useful functional recovery.Introduction
Surgical interventions carry various risks, some of which are common and manageable; others are rare and life-changing. Elective orthopaedic surgery for degenerative joint diseases involve osteotomies where bone is cut and realigned; arthroplasty involves joint dislocation and may potentially lengthen the limb. Arthroscopy may involve limb traction and multiple small portals traversing the periarticular tissues for passage of instruments. Fracture surgery involves operating in limbs with anatomy distorted by injury with disturbance of the normal anatomical planes by haematoma, oedema or scarring, manipulation of bones and insertion of reduction instruments. All may require the use of power tools, instrumentation and insertion of implants. Nerve injuries inevitably occur; but fortunately, the incidence is low. The infrequent occurrence of nerve injuries may result in failure to recognise the key clinical signs in a timely fashion, with consequent delays to diagnosis, referral and treatment.
Nerve injuries cause pain, paralysis, loss of protective sensation and disability. They adversely influence the outcome of the index procedure and may result in litigation, increasing the burden on financially stretched surgical services.
Clinicians will see relatively few iatrogenic nerve injuries in their careers. Surgeons and anaesthetists should be aware of the clinical features and understand that prompt diagnosis and appropriate immediate management often leads to improved functional recovery.[1] Patients with nerve injuries that have been picked up early tend to fare better than those who have a delayed diagnosis of nerve injuries. Delayed diagnosis of nerve injury is associated with chronic neuropathic pain syndromes, peripheral changes including marginal hypersensitivity and cortical remapping with central sensitivity sation and increased risk of complex regional pain syndrome type 2. Skin trophism with loss of protective sensation and sweating may result in ulceration and infection. Chronic paralysis leads to wasted muscles with fatty infiltration, musculotendinous shortening, joint contractures and deformity.
The reasons for delayed diagnosis include failure to recognise the clinical signs, inadequate examination, poor understanding of the pathophysiology of nerve injury, overdiagnosis of neurapraxia, inadequate follow-up once a nerve injury is diagnosed, over-reliance on neurophysiological investigations[2] with little understanding of their limitations, failure to understand those nerve injuries may deepen and that many cases correctly labelled as neurapraxia may require surgical intervention.
This review aims to increase the awareness of iatrogenic nerve injuries relevant to orthopaedic surgery and provide guidance for early recognition and guidance for management.
Mechanism of Injury
In orthopaedic trauma surgery, defining the time of nerve injury is critical and may have important medicolegal implications.[3] Nerves may be injured at the time of the index trauma due to penetrating wounds, laceration from fracture fragments, traction, deformity or compression. At presentation, a comprehensive neurological and vascular examination is mandatory; however, it may be challenging due to pain, deformity, conflicting priorities due to other injuries and altered consciousness. Objective measures of loss of sweating and erythema in a cutaneous territory are features of autonomic sudomotor and vasomotor paralysis most commonly associated with axonotmesis or neurotmesis injury.
The examination findings must be documented meticulously. In the presence of a suspected vascular injury, the limb should be anatomically realigned and then the neurovascular status reassessed. Inadequate perfusion should prompt vascular imaging with a view to emergent fracture stabilisation, vascular exploration and reconstruction as well as consideration of limb fasciotomies.
A deterioration in neurological status after fracture reduction and cast immobilisation or splint application should cause concern. This scenario is common in the management of humeral fractures where the radial nerve function may be lost after reduction. There is published evidence to suggest that many of these injuries will recover well without intervention; however, there is a risk of what could be a true neurapraxic conduction block deepening to become a high-grade axonotmesis if the radial nerve remains entrapped in a fracture, strangulated at the lateral intermuscular septum or compressed by haematoma.[4] Repeated clinical examination will highlight those injuries that are concerning and should then prompt exploration, nerve decompression and fracture stabilisation.[1]
Surgical stabilisation of fractures may involve closed reduction and percutaneous wiring, application of external fixators or intramedullary nailing. Anatomical landmarks may be masked by swelling, and safe corridors may be breached resulting in nerve injury. The use of power tools risks direct nerve injury, thermal injury, tether from nearby soft tissues or complete avulsion. Neurovascular bundles can be entrapped or tethered within fractures. The paediatric supracondylar fracture is an example where the median nerve and brachial artery may be entrapped in the fracture and the ulnar nerve is at risk from wire placement.
Internal fixation of fractures requires exposure with surgical incisions. Incorporating the wounds from open fractures for debridement may lead to inadvertent injury due to unfamiliar anatomy, loss of normal tissue planes and masking of normal landmarks from fracture deformity and haematoma. Standard orthopaedic exposures[5] utilise internervous planes. The bone is exposed by approach between muscle groups supplied by different peripheral nerves. The neurovascular bundles are not routinely exposed. The surgeon should be aware of peripheral nerve anatomy and be prepared to explore the neighbouring peripheral nerves at the time of fracture reduction.
Fracture reduction may require traction and insertion of clamps around the bone to facilitate manipulation. The nerves are at risk if soft tissues are interposed in the jaws of bone clamps.
Temporary fracture stabilisation may be achieved with wires, cerclage wires and clamps. Lag screw fixation may require a drill trajectory that poses a risk of injury to nearby nerves. Plate and screw stabilisation require broad bone exposure and drilling through bone. The soft tissue retraction can cause a direct compression injury or indirect traction injury. The ends of the plate may be poorly visualised, and there is a risk of nerve entrapment under the plate due to tethering of soft tissues adjacent to neurovascular bundles that are normally mobile. The radial nerve is at risk in anterolateral and posterior approaches to the humerus. The drill tip is rarely seen, and caution should be exerted when the trajectory is close to neurovascular structures such as drilling the clavicle for plate application.
Cortical screws may need a bone tap, and these are sharp instruments that can cut nerves at the distant cortex. Taps should never be used on a power driver. Modern screws include locking, self-drilling and self-tapping subtypes. When inserted, care should be taken to ensure the correct length is selected. There is no feedback to warn soft tissue tether. Protruding tips have sharp edges at the drill and tap leading edges that can cause trauma to adjacent nerves.
Fasciotomy for traumatised limbs or for the management of post-revascularisation swelling is infrequently performed, and there are risks from the surgeon not being familiar with the anatomy. The superficial peroneal nerve is at risk in the distal lateral incision in the lower leg. The saphenous nerve is at risk in the medial incision.[6]
During reduction of traumatic joint dislocations, neurovascular bundles may sustain traction injuries. The risk is greatest when excessive force is applied, the incorrect technique is employed or delayed reduction results in soft tissue tether, which can worsen the nerve injury. Consideration should be given to open reduction with mobilisation of nerves in delayed reduction, particularly when there is already a prereduction neurological injury.
Arthroplasty surgery is usually routine, and fortunately, nerve injuries are relatively uncommon and typically of a low grade with spontaneous recovery. The introduction of minimally invasive approaches is associated with higher rates of nerve injury. Complex arthroplasty includes skeletal dysplasia, revision surgery and endoprosthetic replacement for tumour reconstruction. The rates of neurological injury are higher in these cases due to wider exposures, abnormal anatomy, excessive traction, intraoperative bleeding, prolonged dislocation and limb lengthening.
In arthroplasty of the knee and ankle, the tibial nerve and popliteal vessels are at risk of injury during tibial bone resection at the knee and the tibial nerve and posterior tibial artery at risk during resection of the tibial bone at the ankle.
Osteotomies may be employed for post-traumatic deformity correction, congenital deformity correction or for realigning the mechanical axis of a limb for the management of early degenerative joint disease. Nerves are at risk of direct injury from saw blades and drills or indirect traction, compression of the tether from joint realignment and haematoma.
Arthroscopy involves joint distraction, distention, creation of multiple ports for instrumentation and occasionally combination of intra-articular and extra-articular interventions. Anatomical landmarks may be distorted by swelling and soft tissue extravasation of irrigation fluids. Infrequent procedures pose a greater risk of nerve injury. The development of minimally invasive reconstruction capabilities has increased the number of procedures and procedure complexity for these infrequent interventions and poses a risk to nerves. Triangular fibrocartilage repair in the wrist carries a risk of injury to the dorsal branch of the ulnar nerve. Lateral meniscus repair at the knee may risk direct injury or tether of the common peroneal nerve. The arthroscopic Latarjet procedure in the shoulder risks injury to the infraclavicular plexus. Arthroscopic stabilisation of the lateral clavicle risks injury to the plexus and the supraclavicular nerves. The posterior interosseous nerve is at risk in arthroscopic procedures for lateral epicondylitis or impingement in the elbow.
All procedures carry risks of cutaneous nerve injury, traction injury to nerves, diathermy burns and risks of compression from bleeding or post-operative swelling. Tourniquet use on limbs is associated with nerve injuries. Pneumatic tourniquets have controlled pressure, and if well placed, appropriately pressurised, adequately padded and time-limited, the risk of nerve injury is small. The use of a tourniquet allows safe exposure in limbs and probably reduces the risk of iatrogenic injury that accompanies surgery with surgical field obscured by bleeding.
Many orthopaedic procedures are performed with regional anaesthesia alone or as an adjunct to general anaesthesia. Nerve injury may be associated with injection of local anaesthetic around nerves from tamponade, compression from haematoma or scar, intraneural injection, direct fascicle transection from the needle bevel or damage to longitudinal vasa nervora. Blocks should be completed with ultrasound and nerve stimulation. The greatest risk of injury is from incomplete block top up at the same site where stimulation thresholds become unreliable and perineural planes are distorted. In such situations, intraneural injection can readily occur. Performing regional blocks with the patient under general anaesthesia is another risk. There is no pain response to warn of potential nerve injury, and neuromuscular paralysis may render stimulation unreliable. Blocks may mask the pain in compartment syndrome and should be used with caution in trauma surgery.
Classification of Nerve Injury
Understanding the severity of nerve injury enables provision of a prognosis, directs surgical management and allows comparative research into reconstructive techniques. The classification described by Seddon[7] is in common usage but was expanded by Sunderland[8] to provide an explanation of the variable outcomes from axonotmesis injury. Further expansion by Lundborg[9] provides a greater understanding of neurapraxic or conduction block injuries.
Neurapraxia is a conduction block injury without axon damage. There is potential for a full recovery as long as the nerve has a healthy environment and there is no persistent compression. The injury typically involves greater dysfunction in the large energy-dependent myelinated fibres for fast pain perception, light touch, temperature and motor function. The proprioceptive, pressure and smaller non-myelinated autonomic and slow pain fibres are generally preserved. Mild conduction block follows ischaemia or oedema from injury and compression. Severe conduction block injuries have segmental damage to the myelin sheath, which must be replaced before conduction is restored. The higher grade injuries typically resolve completely within 2–3 months from injury.[10]
Axonotmesis injuries have axon damage with Wallerian degeneration of the distal stump due to loss of connection to the cell body and inability to maintain its cell membrane integrity. The degree of supporting connective tissue injury is variable and dictates recovery potential. Low-grade axon injuries have intact endoneural tubes; therefore, recovery potential is good. More severe damage to the perineurium risks neuroma-in-continuity formation and incomplete recovery. Axonotmesis injuries may require surgery for diagnosis, reconstruction or neurolysis.[11]
Neurotmesis injuries are so severe that the nerve trunk is disrupted or there is such severe disruption of the internal nerve connective tissue architecture that a neuroma-in-continuity results without any axon regeneration. These injuries all require surgical restoration of nerve continuity for any functional recovery.
Mixed nerve injuries are those where there are different degrees of severity to different fascicles within a nerve trunk. This can include the partial transection injury from a laceration.
Neurophysiology studies will demonstrate no distal conduction in complete injuries with axonal degeneration (axonotmesis and neurotmesis) after 10 days when Wallerian degeneration is established. In such cases, the electromyography (EMG) will demonstrate increased activity of needle insertion to the muscle, fibrillation and positive sharp waves.
Neurophysiology tests may be falsely reassuring in the early phase after injury. Neurapraxic injuries can subsequently deteriorate if compression is not relieved and so axonal deterioration may be delayed. Traditionally, neurapraxic injuries are described as recovering fully without surgical intervention. Unfortunately, correct diagnosis of a neurapraxia injury without adequate surveillance and incorrect labelling as a neurapraxia injury are two errors in management that may result in poorer outcome after nerve injury. Orthopaedic surgeons must be aware of these common pitfalls.
Clinical Assessment of Nerve Injury
In the rare setting where a nerve injury is identified intraoperatively, the patient should be discussed with the regional nerve injury service. Rarely, a situation arises where the nerve injury can be managed best by dictating the method of fracture fixation. In the setting with radial nerve injury during humeral fracture fixation, shortening of the humerus and plating may allow direct repair of the nerve following debridement. Debridement is not needed in sharp transection; however, crush avulsion, drill and reamer injuries create a large injury zone, and without shortening the bone, the nerve gap would require grafting with inevitably poorer results. Limb shortening is not applicable in the lower limb unless the patient is non-ambulatory predating the injury. Tagging the nerve with a non-absorbable suture will help in identifying the nerve in subsequent re-exploration. Neuropathic pain is the hallmark of a high-grade nerve injury with axon damage and risk of further deterioration. Orthopaedic surgeons should have a high index of suspicion when evaluating patients with nerve pain.
Following a nerve injury, there will be complete or partial loss of motor, sensory and autonomic function distal to the site of injury. The degree of autonomic dysfunction is associated with the injury severity. Conduction block injuries preferentially affect the myelinated axons, and autonomic dysfunction is not seen. In an injury with axonal damage, there will be a deeper flaccid paralysis, loss of pressure awareness, loss of proprioception and all other sensory modalities. All nerve fibre subtypes are involved, and there is dry skin from loss of sudomotor function and erythema in the nerve cutaneous territory from loss of vasomotor tone.
Neuropathic pain is a feature of axonal damage or neurapraxic injuries at risk of further deterioration. Tinel's sign is positive in such situations and is elicited by gently tapping along the course of the injured nerve in a proximal direction. A positive sign is eliciting pain and dysaesthesia in the cutaneous distribution of the nerve when tapping at the site of injury. This should be marked with a cross on the skin, and the position is recorded for subsequent surgical exploration.
These signs are visible acutely and should be carefully evaluated and documented. In late presenting cases, there may be muscle wasting in axonal injuries with trophic changes visible in the denervated skin [Table - 1].

When examining cases at this stage, one should look for a second distal Tinel's sign that will demonstrate some recovery and therefore at least partial continuity of the nerve sheath. The relative strength of each Tinel's point may indicate the likelihood of a favourable outcome. The distance between the Tinel's points can provide a guide to the rate of neural regeneration. Each site should be noted with reference to a bony landmark for subsequent monitoring if an expectant policy is adopted. Rates of regeneration of 2–3 mm per day are seen in low-grade axonal injuries where the injury site is close to the cell body. High-grade injuries have lower rates of progression at 1 mm per day, and static Tinel's sign indicates a neuroma-in-continuity, ruptured nerve trunk or continuity lesion with extrinsic scar or compression that is compromising regeneration.
During the recovery phase, a regenerating nerve may be swollen due to the size of the growth cone, and auto-entrapment at anatomical constriction points may slow or halt Tinel's progression necessitating decompression.
Muscle tenderness is the first sign of reinnervation in continuity lesions and predates visible contraction by a few weeks.[12]
Neurophysiology is of limited use in monitoring recovery from nerve injury. In cases where a time-critical reconstruction may need to be undertaken, early evidence of reinnervation in EMG may predate clinical signs and encourage further monitoring. The signs of reinnervation on EMG include polyphasia and volitional activation.
When a transected nerve is suspected, the nerve should be emergently explored. Most cases are less clear, and there is a tendency to underestimate the likelihood or injury and the severity of injury by the operating surgeon who may not have the knowledge, experience or skills to evaluate a nerve intraoperatively at re-exploration. These reasons and the potential risk of introducing infection to an implant through re-exploration are contributory to late diagnosis and referral. A more useful approach is to document the clinical findings and refer to a regional peripheral nerve injury service. When a nerve injury is identified, a referral should be promptly made by the surgeon who performed the procedure with a transfer of clinical records including pre-operative notes and imaging, operative and anaesthesia records, post-operative records, imaging and prescription charts. This excludes the initial surgeon from the decision-making process, which encourages objectivity and ensures that an independent second opinion is acutely sought by someone capable of nerve exploration, assessment and reconstruction, should it prove necessary. Adjunct imaging of the injured nerve may be helpful to look for haematoma compression but is best arranged at the regional centre where there will be expertise in ultrasound neurography in the setting of injured nerves. Computed tomography may be helpful to look at screw prominence in arthroplasty and fracture surgery when plain radiographs are inconclusive.
Adequate analgesia medication should be provided, and neuromodulatory medications may be commenced before referral if indicted. At a regional centre, nerve blocks may be employed to improve peroperative pain control for injured nerves at re-exploration. Indwelling nerve catheters can be surgically sited close to the injured nerve of regional catheters place proximally with ultrasound guidance.
Secondary Exploration
Re-exploration is recommended when there is a suspected nerve injury that will require reconstruction, in the presence of a nerve with deterioration, if there is uncertainty regarding the diagnosis, severe pain or failure to improve in line with expectation when a monitoring approach has been adopted [Table - 2].

The surgeon undertaking the re-exploration should be a specialist in peripheral nerve surgery. Nerve surgeons from an orthopaedic background have detailed understanding and technical capability if fracture fixation must be adjusted or revised to deal with a nerve injury. In nerve injury complications following arthroplasty, we would recommend that an appropriate arthroplasty surgeon is available in case a revision of the implant is required. This is an unusual scenario; however, we have seen prominent acetabular cups tenting the sciatic nerve posteriorly and glenoid components with screws irritating the posterior cord of the brachial plexus. In such rare situations, revision of the arthroplasty is mandated to try and improve the nerve injury and reduce the neuropathic pain.
Whenever possible, a tourniquet aids exposure without bleeding in distal limb surgery. No neuromuscular blockade should be used, and intraoperative nerve stimulation must be available.
The surgeon should be prepared for nerve decompression, neurolysis, use of barrier wrapping to prevent further scar, nerve grafting with autologous or processed allograft and distal salvage nerve transfers in high-grade proximal injuries where a reliable distal return of function is not guaranteed.
The treating nerve specialist will be able to assess the injury severity, reconstruct where necessary and monitor recovery. In poor outcome cases, the treating surgeon can offer salvage reconstruction with tendon and nerve transfers for paralysis.
Not all cases of nerve injury will be identified acutely. Cutaneous neuromas are a source of pain and could explain a suboptimal result from surgery. They should be considered in all cases of unexplained pain after orthopaedic surgery. Referral to a nerve injury specialist familiar with the peripheral neuroanatomy and common sites of injury will be able to offer guidance. Typically, ultrasound-guided nerve blocks can be used to target nerve implicated in the pain and direct further investigation and management. Common sites of unexplained pain include damage to the infrageniculate branches of the saphenous nerve causing anterior knee pain after open or arthroscopic surgery, the sural nerve may be injured with Achilles tendon surgery, the superficial radial nerve may be injured in De Quervain's release and the palmar branch of the median nerve may be injured during volar surgical exposure of the distal radius. Often, these injuries go unrecognised, but all are familiar to the peripheral nerve specialist who treats these complications regularly.
Conclusion and Recommendations
Although uncommon, peripheral nerve injuries have important consequences for patients with pain, sensation loss and paralysis. Delays to diagnosis and treatment increase the chances of a chronic neuropathic pain syndrome, reduce the chances of functional recovery and increase the cost of litigation settlements.
In 2017–2018, there were 10,673 clinical claims against the UK National Health Service with orthopaedic surgery being the second largest claim by specialty at 12% of the total. Orthopaedic surgery was the leading specialty the preceding year.[13] Between 2003 and 2017, more than 2.5 million arthroplasty procedures have been performed in the UK. More than 80% of those procedures were hip and knee replacements.[14] Hip arthroplasty is the most common orthopaedic procedure resulting in litigation with an estimated incidence of 3 in 1000 cases.[15]
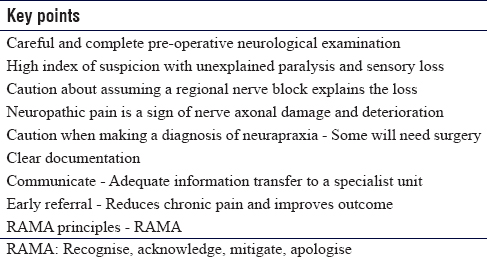
Building on the British Orthopaedics Association Standards for Trauma 5,[16] our recommendations are summarised in [Table - 3].
Ethical considerations
The article is a review based on the current clinical practice of the senior author, current literature and BOAST 5 guidelines. The paper did not require ethics board review.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Author's contribution
JS conceived and designed the article, wrote the initial manuscript, provided research materials, and edited the final draft. JH wrote the initial manuscript and provided research materials. DP conceived and designed the article, wrote the initial manuscript, provided research materials and edited the final draft. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Birch R. Iatrogenous lesions of nerves and arteries in the leg and foot. Foot Ankle Surg 2008;14:130-7. [Google Scholar] |
| 2. | Khan R, Birch R. Latropathic injuries of peripheral nerves. J Bone Joint Surg Br 2001;83:1145-8. [Google Scholar] |
| 3. | Casali MB, Blandino A, Del Sordo S, Vignali G, Novello S, Travaini G, et al. Alleged malpractice in orthopaedics. Analysis of a series of medmal insurance claims. J Orthop Traumatol 2018;19:7. [Google Scholar] |
| 4. | Alvites R, Caseiro AR, Pedrosa SS, Branquinho MV, Ronchi G, Geuna S, et al. Peripheral nerve injury and axonotmesis: State of the art and recent advances. Cogent Med 2018;5:1-45. [Google Scholar] |
| 5. | Hoppenfeld S, de Boer P, Buckley R. Surgical Exposures in Orthopaedics: The Anatomic Approach. 5th ed. Philadelphia, United States: Lippincott Williams and Wilkins; 2016. [Google Scholar] |
| 6. | Moawad MR, Masannat YA, Alhamdani A, Gibbons CP. Nerve injury in lower limb vascular surgery. Surgeon 2008;6:32-5. [Google Scholar] |
| 7. | Seddon HJ. Three types of nerve injuries. Brain 1943;66:237. [Google Scholar] |
| 8. | Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain 1951;74:491-516. [Google Scholar] |
| 9. | Lundborg G. Nerve Injury and Repair. New York: Churchill Livingstone; 1988. [Google Scholar] |
| 10. | Wilbourn AJ, Evans RW. Iatrogenic nerve injuries. Neurol Clin North Am 1998;16:55-82. [Google Scholar] |
| 11. | Rasulić L, Savić A, Vitošević F, Samardžić M, Živković B, Mićović M, et al. Iatrogenic peripheral nerve injuries-surgical treatment and outcome: 10 years' experience. World Neurosurg 2017;103:841-851.e6. [Google Scholar] |
| 12. | Lee EY, Karjalainen TV, Sebastin SJ, Lim AY. The value of the tender muscle sign in detecting motor recovery after peripheral nerve reconstruction. J Hand Surg Am 2015;40:433-7. [Google Scholar] |
| 13. | NHS Resolution. Annual Report and Accounts 2017/2018. NHS Litigation Authority. Report number: HC 1251, 2018. [Google Scholar] |
| 14. | National Joint Registry. 15th Annual Report 2018. National Joint Registry; 2018. [Google Scholar] |
| 15. | Moore AE, Zhang J, Stringer MD. Iatrogenic nerve injury in a national no-fault compensation scheme: An observational cohort study. Int J Clin Pract 2012;66:409-16. [Google Scholar] |
| 16. | British Orthopaedic Association. Standards for Trauma (BOAST) 5: Peripheral Nerve Injury. British Orthopaedic Association; 2012. [Google Scholar] |
Fulltext Views
7,224
PDF downloads
2,520





