Translate this page into:
Management of the scarred nerve using porcine submucosa extracellular matrix nerve wraps
Corresponding Author:
Dominic Power
Birmingham Hand Centre, 6th Floor, Nuffield House, Queen Elizabeth Hospital, Mindelsohn Way, Edgdbaston, Birmingham, B15 2WB
UK
dominicpower1@gmail.com
| How to cite this article: Jordaan PW, Uhiara O, Power D. Management of the scarred nerve using porcine submucosa extracellular matrix nerve wraps. J Musculoskelet Surg Res 2019;3:128-133 |
Abstract
Objectives: Scar tissue formation around the peripheral nerves causes nerve compression and ischemia, but it also causes adherence of nerves to the surrounding tissues, decreasing the nerve's ability to glide, and therefore, causing neurostenalgia – nerve pain with motion due to tether. Our unit has been using a porcine submucosal extracellular matrix (AxoGuard® AxoGen Inc., Alachua, FL, USA) nerve wrap to prevent nerve scarring. The aim of this study is to present a case series of our use of the AxoGuard® and early follow-up data. Methods: This study describes the use of AxoGuard® nerve protectors, including the indications, anatomic locations, and complications. After obtaining ethics approval from the Institutional Audit Review Board, a retrospective review was performed of all cases where AxoGuard® nerve protectors were used from June 2015 to July 2018. Results: Over a 3-year period, AxoGuard® nerve wraps were used in 71 cases. The indication for surgery was a scarred nerve after trauma surgery in 32 cases, scarring after primary nerve surgery in 19 cases, primary trauma in 9 cases, nerve scarring after elective nonnerve surgery in 5 cases, and nerve tumors in 5 cases. There have been no complications directly related to the use of the AxoGuard® nerve protector and no cases of postoperative infection. Conclusions: The AxoGuard® nerve protector has many clinical indications, an excellent safety profile with no reported complications directly related to the nerve wrap, and is effective in mitigating the effects of neurostenalgia following revision neurolysis.Introduction
Scar tissue formation in the vicinity of a peripheral nerve causes compression, ischemia, impaired glide, and tether, resulting in a pain syndrome – neurostenalgia. Prevention of scarring is essential as a nerve requires a healthy surrounding tissue bed to allow free nerve gliding. An unhealthy scarred nerve is often encountered in revision surgery for compressive neuropathies and during neurolysis following Non-nerve and nerve trauma. It is important to consider the potential for epineural scar formation following acute nerve trauma and following surgery for peripheral nerve sheath tumors where the epineurium is sometimes damaged. It is also important to consider the neuroma in continuity with preserved function. In these cases, neuroma excision is not the first line of treatment as it will lead to a loss in function, and neurolysis and wrapping may reduce the impact of tether on nerve pain. Neurectomy and proximal relocation do remain an option in cutaneous neuromas in continuity with a noncritical innervation.[1]
Neurolysis for the scarred nerve poses the problem that surgery to release scarring can lead to recurrent scarring, which will leave the patient without adequate relief. Many surgical techniques have evolved in an attempt to prevent recurrence of scarring, including wrapping the nerve with biological or synthetic barriers.[2],[3],[4],[5],[6],[7],[8] It is, however, important to realize that neurolysis and wrapping or resurfacing only addresses the environment around the nerve and not intraneural scarring that may follow direct nerve trauma and nerve repair.
The aim of this review is to briefly discuss the different surgical options available to deal with scarring around the nerves, with a focus on nerve wraps and specifically to discuss our experience with the use of a porcine submucosal extracellular matrix nerve wrap, the AxoGuard® nerve protector (AxoGen Inc., Alachua, FL, USA) in a tertiary referral peripheral nerve service.
Surgical management
Before proceeding to surgical management, conservative strategies should be employed where possible. In the case of compressive neuropathies, it is important to exclude the possibility of incomplete release, or concomitant compression at other points, for example, a proximal median nerve compression under pronator teres in the case of a failed carpal tunnel decompression, or even incorrect diagnosis, such as radiculopathy.[9]
The aim of revision decompression surgery is to release any compression of the nerve, mobilize the nerve from scar, and wherever possible to create a healthy tissue bed around the nerve, which provides improved vascularity and glide for the nerve and thus decreases the chances of recurrent scarring. This can be achieved by utilization of local flaps, barrier wraps,[6] or transposition of the nerve, specifically in cases of revision cubital tunnel syndrome.
Indications for resurfacing or barrier wraps include recurrent symptoms following surgery for compressive neuropathies, perineural scarring following nerve repair or trauma (such as peroneal nerve injury following knee dislocations and the ulnar nerve following open reduction and internal fixation of distal humerus fractures) and in the cases of a symptomatic tethered neuroma in continuity with preserved distal function. It should also be considered in the setting of acute trauma with a poor surrounding tissue bed or traumatic epineural loss and may even be considered after the excision of peripheral nerve sheath tumors when epineural damage may render the nerve prone to tether at specific anatomical sites.
The ideal barrier should have minimal chance of rejection or inflammation, allow revascularization, restore normal epineural tissue, should allow diffusion of nutrients without allowing axonal sprouting, avoid recurrent scarring, avoid ischemia, and have minimal or no donor-site morbidity.[3],[8]
There are various surgical options, but revision nerve surgery should be regarded as a high-risk surgery. The nerve can be encased in such dense scarring that the nerve cannot be clearly identified, risking injury to the nerve.[9] In such cases, the most important factor is to locate the nerve outside the zone of scarring and carefully dissecting into the zone of scarring. Proximal-to-distal dissection is recommended whenever possible as it is much safer, avoiding dissection into the axilla of nerve branches with the risk of inadvertent sectioning of nerve branches. One should also know when to decide to abort complete neurolysis and leave a cuff of scar tissue around the nerve to avoid nerve damage and when epineurotomy is required with internal neurolysis.[9] The surgeon should be prepared to excise and reconstruct a nerve gap when the surgical findings indicate this approach.
Neurolysis
A simple neurolysis in cases of revision surgery for compressive neuropathies is unlikely to be any more successful than the primary surgery[4],[8] but may be of benefit in cases where an incomplete release is suspected. It could also be of benefit in trauma cases such as knee dislocations and in patients who present with nerve symptoms following non-nerve surgery. In specifically the case of revision cubital tunnel surgery, it can be combined with a transposition of the ulnar nerve.[9],[10],[11],[12]
Autologous vein wrap
An autologous vein graft is harvested and the vein is incised longitudinally. With the intima against the surface of the nerve, the vein is wrapped around the nerve in a spiral fashion and sutured into position.[3],[6],[8] If the vein diameter is larger than the nerve, the vein does not need to be wrapped but can be sutured along the longitudinal cut.[6] Disadvantages include donor-site morbidity, increased operative time,[8] nerve ischemia, recurrent scar formation, and should the saphenous vein be used as a donor, general anesthesia for upper limb nerve surgery. In a case series of revision carpal tunnel decompression, all patients reported an improvement in symptoms and two-point discrimination and electrodiagnostic studies improved following vein wrapping of the median nerve.[3] In a prospective randomized study comparing in situ decompression to decompression and vein wrapping in secondary cubital tunnel syndrome following distal humerus fixation, the vein wrap group showed improvement in sensory outcomes only.[13]
Flap cover
Adipofascial or muscle flaps have been used to create a healthier and more vascularized environment around the nerves to prevent recurrence of scarring.[8],[14],[15] In a series of 14 patients where the Becker flap or anterior forearm fascial flap was used to treat a painful nerve following forearm median nerve trauma, eight patients had complete resolution of pain. They felt that local flaps are better than free flaps, because they are less bulky, have less chance of failure, and have less donor-site morbidity.[15] Local flaps still cause a larger area of scarring and can leave patients with a bulky forearm wound. The hypothenar fat pad flap has been used in cases of recalcitrant carpal tunnel syndrome with excellent results.[14] The abductor digiti minimi flap has been used in patients with complex regional pain syndrome following carpal tunnel release resulting from incomplete release or nerve damage and has been shown to provide reliable results with minimal donor-site morbidity.[16]
Commercially available nerve wraps
There are many different nerve wraps available commercially.[4],[5],[7],[8],[17] Collagen nerve wraps consist of Type 1 collagen, with the most commonly used source being bovine tendons and it degrades between 4 and 8 months.[8] A small series of cases of revision carpal and cubital tunnel syndrome using a collagen wrap showed an improvement in symptoms in 89% of carpal tunnel cases and 83% of cubital tunnel cases.[5]
Chitosan nerve conduits are available as Reaxon® (Medovent, Mainz, Germany). Chitosan is produced by partial deacetylation of chitin, a natural polysaccharide found in the exoskeleton of arthropods, crustaceans, and insect cuticles. This has been studied in humans in acute repair of digital nerves.[18] An experimental study showed that a combination of hyaluronic acid with a chitosan nerve tube inhibited extraneural scarring and adhesions and promoted neural regeneration in rat sciatic nerves.[17]
Human amniotic membrane has been studied in rat sciatic nerves where it has been shown to be effective in recurrent nerve scarring where it has an antifibrotic and pro-regenerative effect.[7] Viable cryopreserved placental membrane (Grafix®-PRIME, Osiris Therapeutics, Inc., Columbia, MD, USA) has also been used and in a series of 7 common peroneal nerve injuries showed some promise.[19]
AVIVE® soft-tissue membrane is a minimally processed human umbilical cord amniotic membrane (AxoGen Inc., Alachua, FL, USA) that is indicated for acutely traumatized nerves to reduce inflammation and scar formation where revision surgery may be required.
Porcine small intestine submucosa has been processed to be used as a porcine extracellular matrix nerve wrap, the AxoGuard® nerve protector (AxoGen Inc., Alachua, FL, USA).[4],[8] The AxoGuard® nerve protector has become our preferred technique for dealing with scarred nerves, for recurrent compressive neuropathies, but also in various other settings to treat and prevent epineural scarring. This will be discussed in more detail during the rest of this article. As part of local clinical governance procedures, we are collecting prospective audit data on the use of the AxoGuard® nerve protector in our unit and will present preliminary data on utilization, safety, and efficacy of the AxoGuard® nerve protector.
The AxoGuard® nerve protector (AxoGen Inc., Alachua, FL, USA)
The AxoGuard® nerve protector is composed of four vacuum-pressed layers of acellular porcine-derived extracellular matrix, sourced from the small intestine submucosa. It is prepared and sterilized using ethylene oxide. It retains its three-dimensional structure while removing cellular components, to prevent immune rejection.[20] It contains both Type 1 and Type 3 collagen.[8] The nerve wrap is rectangular in shape and prerolled into a spiral configuration allowing ready positioning around the cylindrical nerves. The AxoGuard® is supplied in a range of lengths and diameters to a maximum of 10 mm by 40 mm.
The AxoGuard® nerve protector was studied in rabbits. Electrophysiological testing showed no difference between the control and experimental groups. The nerve did not adhere to the wrap, and the nerve could glide easily. Histology showed a healthy nerve with revascularization as early as 1 month following implantation. The nerve wrap appeared to remodel into the connective tissue similar to the nerve epineurium.[20]
There is limited information available on the clinical utilization of the AxoGuard® nerve protector. A series of 12 cases reported that it was safe and effective in the treatment of recurrent cubital tunnel syndrome.[4]
Materials and Methods
This is a descriptive study describing the use of AxoGuard® nerve wraps, including the indications, anatomic locations, and complications.
After obtaining ethics approval from the Institutional Audit Review Board, a retrospective review was performed of all cases where AxoGuard® nerve wraps were used from June 2015 to July 2018. General demographic data, indications, nerves involved, complications, and early follow-up data were collected.
Surgical technique
The nerve wrap has been used for many different indications in various anatomical locations, making it difficult to describe a specific technique, but we will describe some broad principles. Whenever the use of the nerve wrap was considered, it was discussed in detail with the patient preoperatively together with an explanation of alternative strategies. Surgery is performed under loupe magnification. During surgery, the nerve is located proximal and distal to the area of scarring in healthy unscarred tissue. As described earlier, we prefer to perform the neurolysis from proximal to distal through the area of scarring and where necessary a microscope is used to examine the nerve. Once the neurolysis has been completed, the AxoGuard® nerve protector is prepared as per the manufacturer's instructions by soaking it in saline for 5 min and then positioned around the nerve in the area of scarring. Occasionally, it has been necessary to use two wraps in sequence. The wrap is semi-translucent, and therefore, the nerve can be clearly visualized through the wrap, preventing inadvertent suturing of the nerve itself. The wrap is sutured around the nerve with a 6-0 Prolene suture ensuring that it is not too tight, allowing for nerve swelling following the neurolysis and preventing iatrogeneous neo-compression of the nerve. Careful hemostasis is essential to prevent hematoma formation. After skin closure, a bulky dressing is applied, but the early gentle active range of motion is encouraged to enhance nerve gliding.
Results
Over a 3-year period, we have used the AxoGuard® nerve protector in 71 patients. Forty-one were male and 30 were female. The mean age at the time of surgery is 46.2 (17–79) years. Mean follow-up is 5.9 (1–30) months and many patients remain under active follow-up.
The most common indication for the use of the nerve wrap was scarring due to surgery performed for trauma in 32 cases. Two large subsets include scarring following previous nerve repair and for scarring around the ulnar and radial nerves associated with fixation of humeral fractures. Revision compression neuropathy was the primary indication for 19 cases. AxoGuard® was used in nine cases of acute trauma including cases where acute nerve trauma caused epineural loss, common peroneal nerve decompressions following knee injuries, and a case of a nerve transfer following trauma where a considerable size mismatch existed and the nerve wrap was used to prevent axonal sprouting beyond the area of nerve transfer. Five cases were following elective revision non-nerve surgery requiring neurolysis such as neuropathic pain after trigger finger release and Dupuytren's excision. The last group was for tumor excision in five cases, including neurofibromas and schwannomas where there was a large area of epineural damage following excision of the tumor and the anatomical site rendered the nerve liable to scar tether (See [Figure - 1] for summary of indications of nerve wrap).
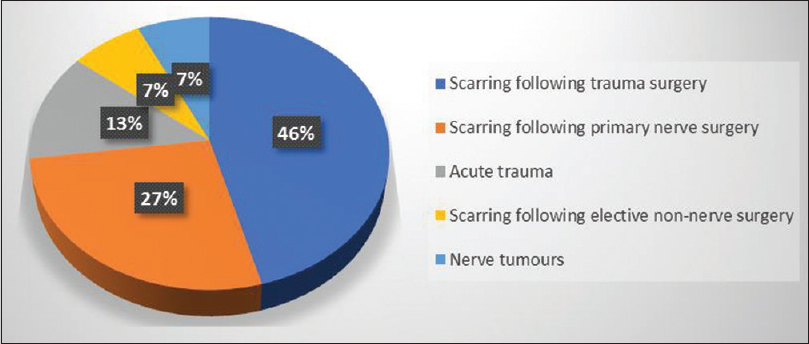 |
| Figure 1: Indications for using AxoGuard® nerve protectors |
The most commonly involved nerve was the ulnar nerve in 32 cases, followed by the median nerve in 14 cases, digital nerves in 11 cases, and the common peroneal nerve in 3 cases. [Table - 1] shows the complete list of nerves involved. Twenty-three cases were used during cubital tunnel decompression, which included recurrent and persistent cubital tunnel syndrome as well as ulnar nerve decompression following distal humerus fixation. Eleven cases were for revision carpal tunnel decompression ([Figure - 2], [Figure - 3], [Figure - 4], [Figure - 5] illustrate clinical use of the Axogaurd® Nerve Wrap).

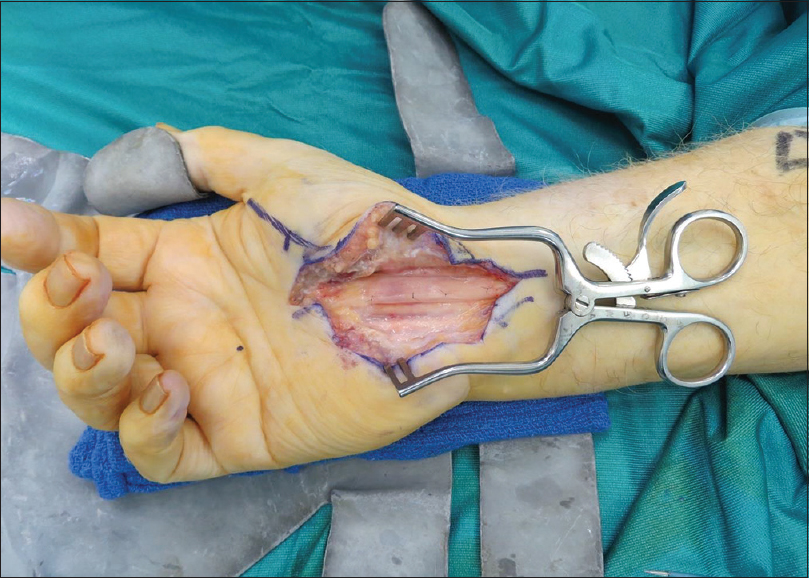 |
| Figure 2: The AxoGuard® nerve protector: Revision carpal tunnel decompression |
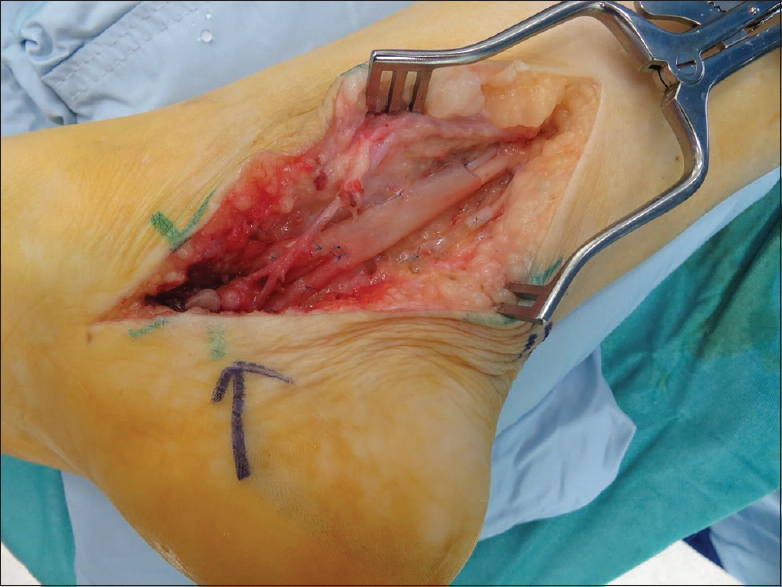 |
| Figure 3: The AxoGuard® nerve protector: Revision tarsal tunnel decompression |
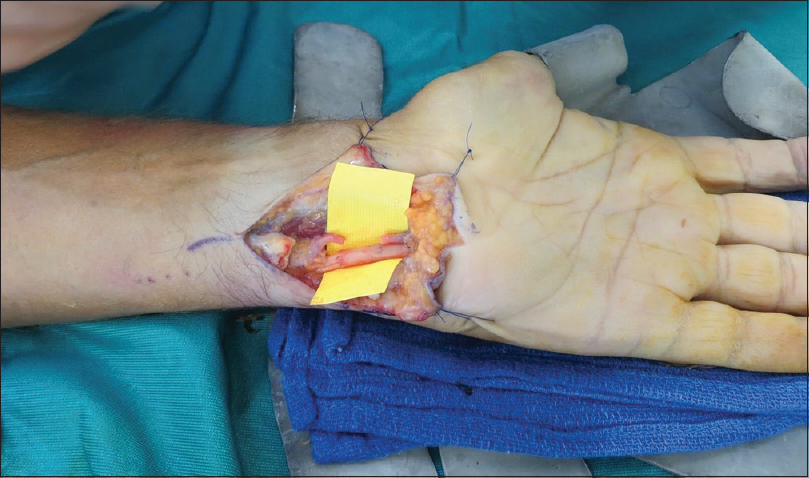 |
| Figure 4: The AxoGuard® nerve protector: Primary ulnar nerve repair with epineural damage |
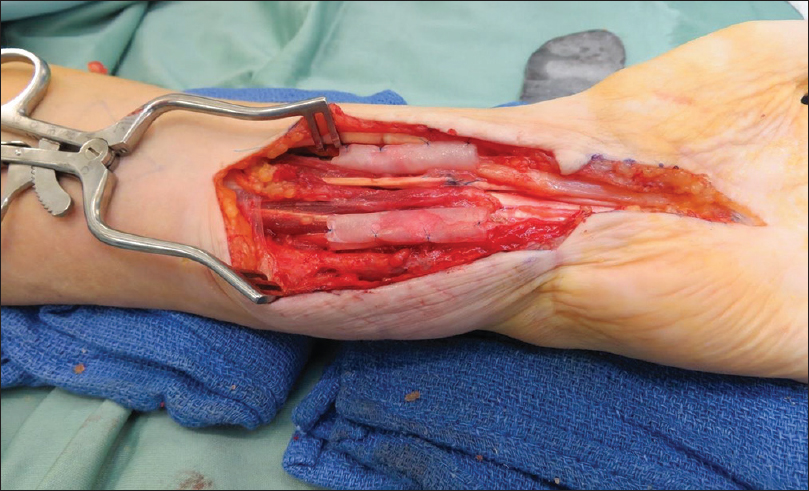 |
| Figure 5: The AxoGuard® nerve protector: Neurolysis median and ulnar nerves following repair |
This is such a heterogeneous group that it is difficult to use a single outcome measure, and as mentioned, this is a descriptive study to illustrate the utilization and safety of the AxoGuard® nerve protector. Approximately two-third of the patients with painful nerve tether report improvements in pain following neurolysis and collagen nerve wrap application. There have been two re-operations. The first was an acute re-exploration 3 days following a cubital tunnel decompression for an acute postoperative hematoma related to anticoagulation. There was an hematoma inside the wrap, and the wrap was reapplied loosely after the evacuation of the hematoma. The second case was a complex case with a long history. Following an injection for a trigger finger, she developed neuropathic pain and finger stiffness. Initial surgery was to perform a tenolysis within the palm and neurolysis of the common digital nerves plus a carpal tunnel decompression. There was evidence of intratendinous myxomatous degeneration at the level of the A2 pulley. The AxoGuard® was placed around the median nerve in the carpal tunnel. Unfortunately, the finger did not regain good movement due to attenuation of the flexor tendon, and the patient developed some neuropathic pain again. The re-exploration was primarily to address the lack of movement. At the time of re-exploration, white deposits were found in the tendon. The area previously wrapped showed a healthy appearing nerve with no adhesions or scarring to the surrounding tissue, but the tendon was in a poor condition, and in the area next to the previous neurolysis, there was a significant scarring noted around the common digital nerves. A first-stage tendon reconstruction was performed, and a new nerve wrap was applied both around the median nerve in the carpal tunnel and the common digital nerves distally. The median nerve demonstrated excellent gliding and a vascular epineurium at the level of the previous wrapping. There was proximal tether at the junction between normal nerve and the previously applied AxoGuard® and so the junctional area was wrapped again at the revision neurolysis. Histology of the excised tendon favored gout rather than steroid deposition.
Discussion
Experimental studies show some promises in preventing scarring around nerves using the AxoGuard® nerve protector.[20] Only a limited number of clinical studies have been reported to date, including surgery for recurrent cubital tunnel syndrome[4] and for lingual nerve repair.[21]
Many different surgical options exist, but none has been shown to be superior in comparative studies. A patient who has developed epineural scarring is already in the difficult to treat group and logic dictates that by performing just a neurolysis, it is likely that the patient will develop recurrent scarring, which is usually symptomatic.[4],[8] Commercially available wraps have the advantage of avoiding donor-site morbidity and reducing theater time. The main issue is availability and cost.
Efficacy is difficult to prove, but to date, clinical studies seem to demonstrate favorable results. A retrospective cohort study comparing lingual nerve repair with and without the use of a collagen conduit found improved functional sensory recovery in the conduit group. In their definition of repair, they included cases that had a neurolysis and wrapping performed.[21] The same senior author proceeded to compare a collagen conduit to the AxoGuard® nerve protector and found no difference in outcomes.[22] In revision decompression of recurrent cubital tunnel syndrome, the AxoGuard® nerve protector was associated with improvement in patients' symptoms in a cohort study.[4] Our study has not been designed to assess efficacy and serves primarily as a database on safety and utilization. With such a heterogeneous group, it is difficult to prove efficacy. It clearly demonstrates that AxoGuard® can be used in many different scenarios and should not be limited to only cases of revision of recurrent compressive neuropathy.
The AxoGuard® nerve protector demonstrates no safety concerns, and to date, we have not seen any cases of infection, persistent inflammation, or recurrent symptomatic perineural fibrosis. Both our cases of re-exploration were unrelated to the nerve wrap itself. It was interesting to note in the second case that the area where we previously had applied the wrap, the nerve appeared macroscopically normal without a tether and showed a healthy nerve. One could argue that we should have extended the wrap over a longer section of the nerve at the index surgery to prevent junctional scar formation in mobile nerve segments.
Shortcomings of this study include the paucity of comparable efficacy data. A prospective study must be undertaken to compare outcomes with and without the use of an adjunctive barrier wrap in a randomized controlled trial (RCT). The selected outcome measure should reflect improvements in nerve function and pain, and a large number of patients will be required to demonstrate efficacy. The major disadvantage of the Axogaurd® nerve protector is cost and to justify its ongoing use, one should ideally prove its superiority over a simple neurolysis by undertaking a RCT. Our unit is in the process of collecting outcome data in the two major groups of compressive neuropathies, namely revision cubital and carpal tunnel decompression, and will be publishing this data separately.
Conclusions
There are many different surgical options to treat the scarred nerve with no comparative trials published to date. The AxoGuard® nerve protector (AxoGen Inc., Alachua, FL, USA) is safe with no complication related to the use of the nerve wrap. It can be used in many different scenarios related to the treatment and prevention of epineural scarring and does not need to be limited to revision of compressive neuropathies.
Ethical consideration
Ethics approval was obtained from the Institutional Audit Review Board, CARMS-14188.
This study adheres to the Declaration of the Helsinki 2013.
Financial support and sponsorship
Nil.
Conflicts of interest
AxoGen Inc. has sponsored training events for staff at the Birmingham Hand Centre, and the Birmingham Hand Centre Research Unit receives funding from AxoGen Inc. for participation in their international registry study on nerve allograft.
Author's contributions
PWJ contributed to literature search, data acquisition, data analyses, manuscript preparation, and manuscript review. OU contributed to data acquisition and manuscript review. DP contributed to concept, design, manuscript preparation, manuscript review, and manuscript edit. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Jordaan P, Wang CK, Ng CY. Management of painful cutaneous neuromas around the wrist. Orthop Trauma 2017;31:290-5. [Google Scholar] |
| 2. | Kokkalis ST, Mavrogenis AF, Vottis C, Papatheodorou L, Papagelopoulos PJ, Soucacos PN, et al. Median nerve biodegradable wrapping: Clinical outcome of 10 patients. Acta Orthop Belg 2016;82:351-7. [Google Scholar] |
| 3. | Sarris IK, Sotereanos DG. Vein wrapping for recurrent median nerve compression. J Am Soc Surg Hand 2004;4:189-94. [Google Scholar] |
| 4. | Papatheodorou LK, Williams BG, Sotereanos DG. Preliminary results of recurrent cubital tunnel syndrome treated with neurolysis and porcine extracellular matrix nerve wrap. J Hand Surg Am 2015;40:987-92. [Google Scholar] |
| 5. | Soltani AM, Allan BJ, Best MJ, Mir HS, Panthaki ZJ. Revision decompression and collagen nerve wrap for recurrent and persistent compression neuropathies of the upper extremity. Ann Plast Surg 2014;72:572-8. [Google Scholar] |
| 6. | Masear VR. Nerve wrapping. Foot Ankle Clin 2011;16:327-37. [Google Scholar] |
| 7. | Lemke A, Ferguson J, Gross K, Penzenstadler C, Bradl M, Mayer RL, et al. Transplantation of human amnion prevents recurring adhesions and ameliorates fibrosis in a rat model of sciatic nerve scarring. Acta Biomater 2018;66:335-49. [Google Scholar] |
| 8. | Dy CJ, Aunins B, Brogan DM. Barriers to epineural scarring: Role in treatment of traumatic nerve injury and chronic compressive neuropathy. J Hand Surg Am 2018;43:360-7. [Google Scholar] |
| 9. | Tang P, Hoellwarth JS, Chauhan A. Recurrent cubital tunnel syndrome: A Critical analysis review. JBJS Rev 2016;4:pii: 01874474-201603000-00007. [Google Scholar] |
| 10. | Ehsan A, Hanel DP. Recurrent or persistent cubital tunnel syndrome. J Hand Surg Am 2012;37:1910-2. [Google Scholar] |
| 11. | van Gent JA, Datema M, Groen JL, Pondaag W, Eekhof JL, Malessy MJ, et al. Anterior subcutaneous transposition for persistent ulnar neuropathy after neurolysis. Neurosurg Focus 2017;42:E8. [Google Scholar] |
| 12. | Grandizio LC, Maschke S, Evans PJ. The management of persistent and recurrent cubital tunnel syndrome. J Hand Surg Am 2018;43:933-40. [Google Scholar] |
| 13. | Sadek AF, Fouly EH, Abdel-Aziz AA, Sayed MA, El-Mahboub NM, Hamdy M, et al. Autogenous vein wrapping versus in situ decompression for management of secondary cubital tunnel syndrome after surgical fixation of elbow fractures: Short-term functional and neurophysiological outcome. J Hand Microsurg 2016;8:38-44. [Google Scholar] |
| 14. | Strickland JW, Idler RS, Lourie GM, Plancher KD. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J Hand Surg Am 1996;21:840-8. [Google Scholar] |
| 15. | Elliot D, Lloyd M, Hazari A, Sauerland S, Anand P. Relief of the pain of neuromas-in-continuity and scarred median and ulnar nerves in the distal forearm and wrist by neurolysis, wrapping in vascularized forearm fascial flaps and adjunctive procedures. J Hand Surg Eur Vol 2010;35:575-82. [Google Scholar] |
| 16. | Cheung K, Klausmeyer MA, Jupiter JB. Abductor digiti minimi flap for vascularized coverage in the surgical management of complex regional pain syndrome following carpal tunnel release. Hand (N Y) 2017;12:546-50. [Google Scholar] |
| 17. | Li R, Liu H, Huang H, Bi W, Yan R, Tan X, et al. Chitosan conduit combined with hyaluronic acid prevent sciatic nerve scar in a rat model of peripheral nerve crush injury. Mol Med Rep 2018;17:4360-8. [Google Scholar] |
| 18. | Bąk M, Gutkowska ON, Wagner E, Gosk J. The role of chitin and chitosan in peripheral nerve reconstruction. Polim Med 2017;47:43-7. [Google Scholar] |
| 19. | Rodriguez-Collazo E, Tamire Y. Open surgical implantation of a viable cryopreserved placental membrane after decompression and neurolysis of common peroneal nerve: A case series. J Orthop Surg Res 2017;12:88. [Google Scholar] |
| 20. | Kokkalis ZT, Pu C, Small GA, Weiser RW, Venouziou AI, Sotereanos DG, et al. Assessment of processed porcine extracellular matrix as a protective barrier in a rabbit nerve wrap model. J Reconstr Microsurg 2011;27:19-28. [Google Scholar] |
| 21. | Erakat MS, Chuang SK, Shanti RM, Ziccardi VB. Interval between injury and lingual nerve repair as a prognostic factor for success using type I collagen conduit. J Oral Maxillofac Surg 2013;71:833-8. [Google Scholar] |
| 22. | Wilson MT, Chuang SK, Ziccardi VB. Lingual nerve microsurgery outcomes using 2 different conduits: A Retrospective cohort study. J Oral Maxillofac Surg 2017;75:609-15. [Google Scholar] |
Fulltext Views
5,420
PDF downloads
708





