Translate this page into:
Pathogenesis, clinical evaluation and non-surgical management of symptomatic neuromas: A literature review
Corresponding Author:
Tom Challoner
Department of Plastic Surgery, Queen Elizabeth Hospital, Mindelsohn Way, Birmingham, B15 2GW
UK
tom.challoner@gmail.com
| How to cite this article: Challoner T, Power DM, Beale S, Nijran A. Pathogenesis, clinical evaluation and non-surgical management of symptomatic neuromas: A literature review. J Musculoskelet Surg Res 2019;3:15-21 |
Abstract
Neuromas are an often-underdiagnosed cause of chronic pain resulting from a peripheral nerve injury. There are two main sub-types as follows: end neuromas result from nerve transection caused by transection of a nerve due to trauma, iatrogenic injury, following amputation or oncological resection and neuroma-in-continuity where axonal injury with loss of internal neural architecture following direct trauma, compression or traction results in disorganised neural regeneration with no loss of physical continuity of the nerve sheath. Pharmacological symptom control is often inadequate. A comprehensive, structured assessment and multi-modality management regimen is required to achieve favourable outcomes in neuroma management. Strategies include peripheral neuromodulation, neurorehabilitation interventions, psychological support, peripheral nerve blockade, radiofrequency ablation and surgery. Surgical interventions are aimed at restoration of nerve continuity when possible, modification of the nerve environment and neuroma relocation or capping. This review will discuss diagnostic techniques and management strategies of peripheral neuromas.
Introduction
Within a peripheral nerve, axons lie in endoneurial tubes and are bound together by perineurium connective tissue into fascicles. Groups of fascicles are then surrounded by a further sheath, the epineurium to form a nerve trunk. Peripheral nerves with mixed sensory and motor modalities contain multiple fibre types, each specifically evolved for a particular function. Large diameter axons (A and B) are wrapped within myelin sheaths formed from Schwann cells. The axon is exposed at small junctions between Schwann cells where voltage-gated channels in the axon cell membrane predominate. These nodes of Ranvier are essential to normal conduction in myelinated axons and are the point at which an action potential depolarises the cell membrane. Rapid propagation along the nerve follows after triggering the next node in sequence to depolarise after reaching the threshold potential required for voltage-gated sodium channel opening through electrolyte conduction in the extracellular fluid. The consequent saltatory conduction is rapid and energy efficient due to limited ion exchange at these depolarisation points rather than depolarisation of the whole-cell membrane that would require additional energy consumption for restoration of membrane resting potential after large ion shifts. The large fibres are sensitive to ischaemia, distortion of the nodes through oedema or to demyelination. Smaller axons (C) are not enveloped in myelin but are supported by Schwann cells. They conduct at slower rates and with lower action potential frequency. They are involved in slow pain transmission and homeostatic autonomic regulation. The smaller fibres are relatively resistant to conduction block due to ischaemia resistance and conduction independent of a myelin sheath. Dysfunction in these fibres follows severe nerve injury with axonopathy or complete nerve transection.
Low-grade nerve injury is defined as a neurapraxia or conduction block and is typified by ischaemia, oedema or demyelination with preservation of axon continuity. Clinical examination demonstrates loss of motor function, sensory disturbance (fast pain, light touch and temperature), but the preservation of sudomotor and vasomotor autonomic function. Joint position sense is often preserved as well as pressure and slow pain (C fibre) function. Tinel's sign is absent at the site of injury unless there is a mixed nerve injury or a high-grade injury with axonal degeneration.[1]
In a high-grade injury, there is axon rupture or nerve trunk rupture [Table - 1] and [Table - 2]. In such cases, the distal axon is disconnected from the cell body and is unable to maintain a membrane potential for more than a few days after which the cell membrane breaks down and the debris is phagocytosed by macrophages. The Schwann sheath reorganises and neurotropic release prepares the local environment for neural regeneration. The proximal axon forms a growth cone with numerous axon sprouts exploring the environment using both mechanical and chemical stimulation to target re-population of endoneural tubes of the post-injury nerve segment.[2]
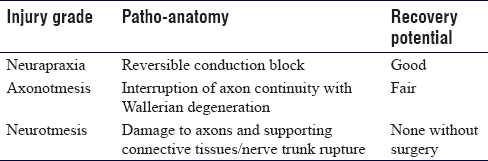

The clinical signs of a high-grade injury include complete loss of all axons within the nerve, including myelinated and unmyelinated fibre sub-types. There is dry skin in the area of cutaneous innervation with erythema from the loss of vasomotor tone. Pain is typical of such injuries with a strong Tinel's sign at the site of injury. A rapidly progressive Tinel's sign advancing at 2–3 mm/day is found in intermediate grade injuries (low-grade axonotmesis) and a slower advancement at 1 mm in a higher grade of axonotmesis. A static Tinel's sign at sequential clinical assessments suggests a high-grade axonotmesis with formation of a neuroma-in-continuity, or a neurotmesis with rupture of the nerve trunk and formation of an end neuroma.
Nerve lesions with interruption require surgical nerve repair after debridement. Surgery should be performed using an operating microscope and microsurgical sutures such as 9-0 or 10-0 are placed in the epineurium to restore alignment without distortion of the fascicle structure, to avoid symptomatic neuroma. A cadaveric study demonstrated the superiority of microsurgical repair compared with the use of loupes, based on alignment, gapping, tension and fascicle extrusion,[2] but only half of the surgeons routinely used microscopes for peripheral and digital nerve repairs. There will always be a neuroma in continuity despite meticulous repair but often without symptomatic pain.
Following debridement, after resection of a neuroma-in-continuity or after resection of an end neuroma following a delay to exploration, there is a segmental loss of nerve tissue, which must be restored. Options include reversed sensory autologous cable nerve grafts or processed nerve allograft to bridge the defect.[3]
Pathophysiology of Neuromas
A neuroma is defined as a disorganised mass of uncontrolled axonal proliferation and connective tissue scar at the site of a nerve injury. Cell phenotypes within the neuroma include fibroblasts, Schwann cells and myofibroblasts, which have contractile properties and cause the collagen matrix to contract around unmyelinated nerve fibres, leading to pain.[4] Extrinsic scar on a nerve with a neuroma produces the clinical pain syndrome of neurostenalgia due to nerve tether or constriction causing ischaemia. There are two broad sub-types of neuromas: End neuromas following transection or rupture of a nerve trunk and neuromas-in-continuity are found in a high-grade axonotmesis injury (Sunderland Grade 4) or at the site of repair of a neurotmesis injury (Sunderland Grade 5). The neuroma-in-continuity occurs in response to internally damaged fascicles, with a meshwork of thickened connective tissue that prevents axon regeneration and growth towards the distal target.[4]
Aetiology of Neuromas
Nerve injuries commonly occur as a result of medical procedures.[5] The mechanism of injury can be traction, direct transection, diathermy burns or compression by implants. The anatomical distortion, obscured vision from bleeding and limb manipulation associated with fracture and trauma surgery renders nerves vulnerable to injury. Arthroplasty surgery with joint dislocation and risk of limb lengthening is another major cause of iatrogenic nerve injury. End neuromas are associated with limb amputations for trauma, tumour surgery, vascular disease and complications of diabetes.
Tensegrity of intact biological tissues results in peripheral nerve retraction after injury. The consequent gap, increased the modulus of elasticity with a delay to repair and debridement of the nerve ends creates a risk of excessive tension at the site of repair that results in ischaemia and promotes connective tissue proliferation, which invades the suture site creating an obstacle for axon regeneration.[6] Clark et al. revealed a reduction in blood flow of 50% with an 8% increased tension in nerve repairs[7] which in turn increases scar formation.[8]
The tension at the site of a nerve repair is concentrated at the suture-epineurium interface and the stress concentration can lead to suture failure, intraneural haemorrhage and further scar formation. Under these conditions regenerating axons may fail to become adequately myelinated at the distal stump and they migrate to the periphery away from areas of stress concentration and neuroma sensitivity ensues.
Prevention of Neuroma Formation
Many techniques have been described for preventing neuroma formation during amputation procedures. These include epineural ligation, capping, microneural anastomosis, bipolar diathermy and chemical treatment with phenol, alcohol and steroids.[9] There is no evidence to support any particular procedure; although, there is renewed interest in capping with the development of bioresorbable polymer caps that may mitigate the historic persistent mechanical irritation of nerve ends from silicone cap treatment. Simple transection under gentle traction allows deeper retraction of the cut nerve end to reduce the risk of scar tether at the site of amputation.[10]
Clinical Diagnosis of Neuromas
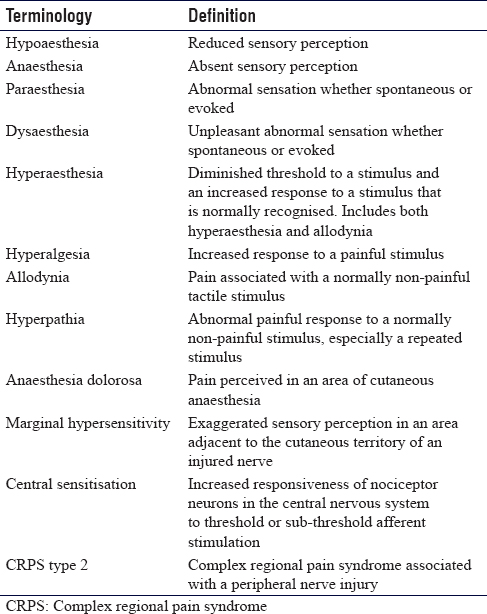
A detailed history and examination are crucial to the diagnosis of a neuroma. Patients will report a sensory disturbance with numbness or hypersensitivity in the cutaneous territory of the injured nerve and pain at the site of nerve injury. Generally, such symptoms are described as burning, pins and needles (paraesthesia), unpleasant sensory perception (dysaesthesia) or electric shocks in the affected limb [Table - 3].[11] Nerve tethering with pain may present as pseudoparalysis due to reluctance to move tendons, joints or skin due to triggering of pain. Long-standing nerve injury is associated with altered temperature regulation and symptoms of cold intolerance. On examination, numbness can be quantified using the 10/10 test, which is useful for monitoring longitudinal responses to treatment.[12] This involves asking the patient to quantify light touch sensation, with 0/10 being complete anaesthesia, and 10/10 being normal sensation. Light touch may be painful (allodynia) and there is a lowering of threshold for perception of painful stimuli (hyperalgesia). Tinel's sign may be elicited at the site of nerve injury. Callahan describes this as 'The most distal point at which the patient experiences a tingling sensation that radiates peripherally in the cutaneous distribution of the nerve is the point of a positive Tinel's sign'.[6] Nerve tether (neurostenalgia) is demonstrated with pain exacerbation on passive movement or stretch. Trophic changes may be observed in distal innervated territories from disuse or denervation. Autonomic disturbance may be associated with the loss of autonomic innervation for complete nerve trunk lesions with a distal loss of sudomotor and vasomotor function. In cases of marginal hypersensitivity, there will be exaggerated pain responses and hypersensitivity in areas adjacent to denervated skin. Central sensitisation may manifest as apprehension and sensitivity on touching any cutaneous area in the limb out with the injured nerve territory.
Pain is associated with axonal injury or progressive nerve deterioration in the acute setting, however, not all complete nerve transections cause on-going pain. The persistence of neuropathic pain is due in part to abnormal mechanical stimulation of the damages nerve and in part due to centrally mediated changes in cortical processing, perhaps due to the loss of normal sensorimotor input and a secondary amplification of sensory stimuli resulting in a risk of marginal hypersensitivity in intact nerve territories surrounding an area of hypoaesthesia or anaesthesia.[13] Following partial injury to a nerve there is increased responsiveness with reduced threshold to stimulation in its receptive field. Peripheral hyperstimulation of predominantly C fibre nociceptive afferents results in central sensory processing changes that produce allodynia, hyperalgesia and hyperpathia.
Complex regional pain syndrome (CRPS) may be associated with a nerve injury (type 2). Patterson et al.[14] extrapolate from the international association for the study of pain guidelines to define CRPS 2 as 'neuropathic pain, caused by peripheral nerve irritation from compression or neuroma, which extends beyond the distribution of the affected nerve, associated with autonomic changes, trophic events and functional impairment.' CRPS type 1 has no identifiable peripheral nerve causation. However, careful review of cases erroneously diagnosed with CRPS type 1 may identify a treatable nerve injury precipitating the syndrome.
In CRPS type 2, there may be autonomic instability, with skin mottling, excessive sweating and disturbed hair and nail growth. A repeated non-painful stimulus may become painful (hyperpathia). Patients living with chronic pain may demonstrate evidence of a reactive depression, which must be diagnosed and addressed if a successful outcome is to be achieved in the management of symptomatic neuromas. Medication history is useful in detailing the current and past drug therapy for pain, neuromodulation and depression and can guide future management in the peri-operative period. Patients should be involved in this decision-making to feel that they have control. Fear of loss of control and being in pain accompanies many patients with neuropathic pain from neuromas. Previous anaesthetic records, operative records and neurophysiology should be reviewed. Regional nerve blocks may rarely be associated with nerve injury due to direct trauma from the stimulator needle, intraneural injection, haematoma or ischaemia from tamponade with high volume injections in tight fascial spaces. Patients with a nerve injury may have a delayed diagnosis due to the masking effects of the regional anaesthetic block or erroneous diagnosis of a block-related injury to explain a deficit following surgery.
Neurophysiology is of limited use in the investigation of a neuroma but may be of benefit in assessing residual function or recovered function after a repair when there is uncertainty whether the surgical strategy should involve neurolysis and wrapping or resection of a neuroma-in-continuity and grafting. Limitations of nerve conduction studies in combination with electromyography are that they are restricted to examining large unmyelinated fibres (Aβ), rather than Aδ of C fibres, which are the actual culprits in neuropathic pain.[15] These studies, therefore, can provide evidence of nerve disease, but negative testing cannot eliminate minor damage to small sensory fibres.
Ultrasound may be useful in some cases to identify the site of a nerve injury and neuroma; however, local sensitivity may render the investigation intolerable for patients unless preceded by a nerve block. Heinen et al.[16] described the technique of neurosonography and reported fascicular ratio as a method of assessment of the severity of nerve injury and regenerative potential. In addition, ultrasound can evaluate the gliding tissues surrounding a nerve.[17]
The field of magnetic resonance (MR) neurography is developing and improved imaging sequences and correlation with clinical and electrophysiological tests may guide whether surgical exploration is warranted in continuity nerve lesions after injury.[18]
Diffusion tensor imaging is a development in MR imaging that has been used to define functional nerve imaging in the central nervous system and shows promise in imaging peripheral nerves. Developments in the peripheral nervous system may be able to detect continuity nerve injuries without distal function or monitor recovery after continuity nerve injury or repair.[19]
Peripheral Nerve Local Anaesthetic Blocks
Local anaesthetic peripheral nerve diagnostic blocks can be performed safely and accurately using ultrasound and nerve stimulation. Considered as part of the clinical assessment pathway they are useful in defining anatomically the nerve origin of the pain and potentially predicting the response to surgical management. They are particularly useful in situ ations where there is considerable peripheral nerve cutaneous field overlap as is found in the superficial radial nerve and the lateral cutaneous nerve of the forearm, in cases where there is marginal hypersensitivity, which can be demonstrated to dynamically respond to the blockade of the injured nerve and in cases of diagnostic uncertainty when there are aberrant anatomical pathways and intraneural connections that transmit the painful stimulus.[20] The utility of targeted peripheral nerve blockade can be improved using sequential blocks with repeated clinical evaluation after each stage, using a visual analogue pain scale after each block and considering ultrasound imaging of the site of suspected nerve injury or neuroma after adequate pain relief following the block [Figure - 1].
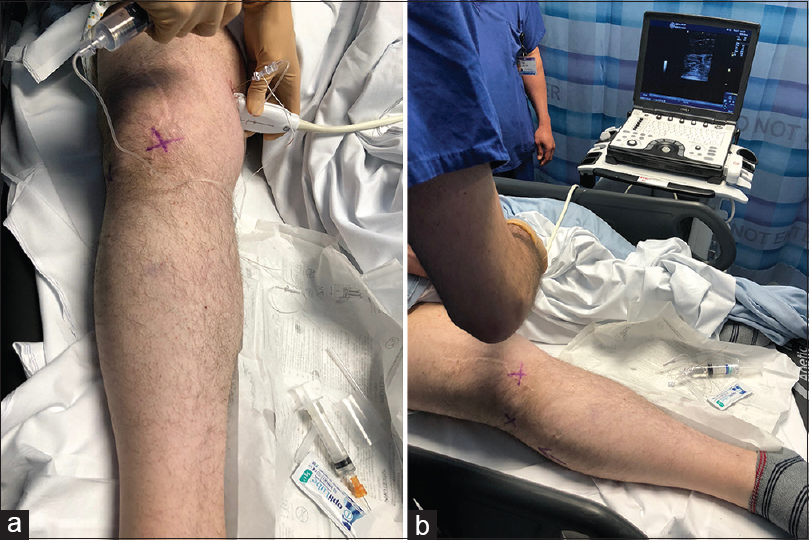 |
| Figure 1: (a and b) Ultrasound-guided local anaesthetic block of the saphenous nerve for diagnosing a geniculate branch neuroma following knee surgery. The maximum Tinel's sign is marked with a cross |
Non-Surgical Management of Neuroma
The ultimate goal in the treatment of a neuroma is to restore feeling and movement in critical nerves when feasible, to improve pain and to restore function. A multidisciplinary approach is warranted with pain specialists, physiotherapists, hand therapists, anaesthetists, radiologists, psychologists and peripheral nerve surgeons working together towards this common goal. A period of non-operative management should be considered in most cases of end neuroma and some cases of suspected neuroma-in-continuity.
Pain management
The management of chronic neuropathic pain is challenging and controlling pain may require combination pharmacotherapy using analgesia and pain modulating medications with antidepressants.[21] Pain specialists are often employed to manage pharmacological treatment of neuropathic pain employing oral and topical agents as well as screening patients for intervention with selective nerve blocks, nerve stimulators and radiofrequency ablation. Oral medications include simple analgesics, opioids, antidepressants and anticonvulsants. Balancing efficacy with an acceptable side effect profile is the challenge. The neuromodulatory effects of the anticonvulsants gabapentin and pregabalin have helped to reduce the use of opioids in the management of neuropathic pain.[21]
A meta-analysis in 2015 by Finnerup et al.[21] supported first-line usage of gabapentin, pregabalin, duloxetine and amitriptyline, with weak evidence for a topical patch or opioid therapy. Adequate long-term pain relief has been reported in only 30%–40% of cases using this strategy in isolation.[22] The use of opiate analgesia in isolation in patients with chronic pain is often unsuccessful. In neuropathic pain management, it can lead to long-term opiate dependence due to increasing doses with little symptomatic benefit.
Two topical agents are currently licenced for neuropathic pain. Due to the limited systemic absorption they have fewer systemic side effects associated with oral medications. Lidocaine 5%, available as a patch or cream and is useful in areas of hyperalgesia and allodynia. It binds voltage-gated sodium channels to block action potentials.[23] Its higher concentration is indicated due to its poor absorption through the dermis. A recent cochrane review found no high-quality randomised trials supporting the use of topical lidocaine, but smaller trials indicated its effectiveness.[24] A recent randomised controlled trial (RCT) proved superiority over oral pregabalin with fewer side effects after 4 weeks.[25]
The other approved topical agent is capsaicin, which is a transient receptor potential vanilloid-1 receptor agonist. It causes a persistent desensitisation of cutaneous nocioceptors[23] and a reversable reduction in nerve fibre density. Patches are designed for a 60-min application under the direct supervision of a pain specialist, after which time they are removed. Mou et al.[26] demonstrated its superiority over a control while long-term monitoring of outcomes by Gálvez[27] showed 31% of patients describe their symptoms as improved or markedly improved.
Observational studies investigating topical clonidine, amitriptyline and ketamine have demonstrated positive results symptomatically over longer periods, with fewer side effects compared to their oral equivalent; however, randomised controlled trials are required to demonstrate optimum dosage and treatment duration.[26]
Therapy
Therapy for neuropathic pain from neuromas should be aimed at both peripheral and central pain modulation.
In the periphery, neural glide exercises aim to prevent scar tether in the vicinity of the neuroma. Active and passive mobilisation exercises should be combined with soft-tissue massage to change mechanical forces acting on a neuroma. Desensitisation exercises target areas of hypersensitivity and involves recalibration of afferent signals through progressive exposure of the affected area to various degrees of pressure and texture. In a study of 39 patients experiencing hyperaesthesia a desensitisation programme showed statistically significant improvements (P < 0.001) in pain/discomfort at rest and with use or touch, a decrease in the size of the sensitive skin area and higher performance in daily occupations.[28] Therapists can advise on the management of trophic areas of skin to prevent thermal or pressure injury. They can support the patient in the rehabilitation journey, setting tailored goals, monitoring recovery, advising on pain management and measuring the outcome.
Neuromas are associated with altered central processing of sensory stimuli and without targeting rehabilitation at these central pathways, peripheral rehabilitation strategies are likely to fail. It is well recognised that within minutes after an injury to the nerve, there is a cortical response with a reorganisation of the sensory brain cortex. The area affected no longer receives any sensory input with adjacent cortical areas expanding. One reason for this may be owing to the long initial period of absent sensibility. This allows functional cortical reorganisation changes to take place due to initially the loss of sensory input and later on misdirected axonal outgrowth.[29] This reorganisation changes the 'cortical hand map'. Based on this theory, the implementation of sensory re-education programmes has long been recognised as a treatment modality following nerve injury, since its first description by Wynn Parry in 1966.[30] A recent RCT in 2015 by Rosen et al.,[31] using early sensory re-education within the first week found that at 6 months, discriminative touch was significantly better in the early intervention group and improvement between 3 and 6 months was also greater in the intervention group. However, they found no significant difference in motor function, pain or in the total score.
Sensory re-education is useful in improving the quality of outcome for patients with partial neuroma-in-continuity after nerve injury or repair where the potential functional losses from excision and grafting are deemed an unacceptable risk where useful function remains. The aim of such strategies is to improve functional use of the affected body part.
Sensory re-education is often described in two phases. Within phase 1 therapy concentrates on maintaining the 'cortical hand map' by using both visual-tactile and audio-tactile stimuli. Within phase 2, when there is evidence of sensory return the programme can be advanced to include a tactile/stereognosis programme, for example, identifying textures and objects with vision occluded.
One therapy intervention used in a sensory re-education programme is mirror therapy. This uses the predominance of the visual pathways to over-ride abnormal somatic afferent input and normalise stimulation thresholds and responses. Blocking the view of the affected limb and observing the reflected images of the unaffected side can allow the therapist to touch the non-affected limb and simulate contact with the affected limb, decreasing apprehension. Contact with the affected limb can be introduced. Mirroring movements enables the restoration of functional motion in patients with pseudoparalysis. The treatment should be commenced with a trained therapist and graduated in terms of intensity and duration, tailored to the patient response.[32] In cases of nerve injury and neuroma resulting in CRPS type 2, mirror therapy is a useful adjunct to other therapy modalities and shows promising results in neuropathic pain management.[33],[34]
Neuromodulation
Peripheral nerve stimulation or neuromodulation can be employed to reduce the intensity of pain in neuroma management. The technique involves the application of an external electrical stimulating probe applied to the affected nerve proximal to the neuroma for short periods of 5–10 min repeated over several weeks. The stimulation can be gradually increased as tolerated. In a series of 102 patients, 30% experienced complete resolution, while 21.5% experienced pain relief lasting for weeks to months, electing for no further treatment.[35] This technique is explained by the gate control theory, first described in 1965 by Melzack and Wall[36], which explains that stimulation of Aβ non-nociceptive fibres can interfere and inhibit the transmission of nociceptive signals, reducing pain.
Psychological support
Patients living with chronic neuropathic pain will experience negative psychological, social and vocational effects. Chronic pain has a significant emotional component and patients may have impaired resilience, sleep disturbance, anxiety, hypervigilance or depression. Psychology support including counselling and cognitive behavioural therapies can help patients understand these negative effects and develop coping strategies. Mindfulness, meditation and hypnosis are useful strategies for managing pain. Patients must be able to engage with psychological therapy for it to be successful. Unfortunately, there remain negative connotations associated with a diagnosis of a mental health disorder and patients can wrongly assume that such a diagnosis raises doubt in the mind of a treating clinician regarding the presence of a physical explanation for the pain. A sensitive and supportive approach as part of a multi-professional team is key to patient engagement, a successful outcome from therapy management and preparation for surgery when indicated.
Conclusion
Damage to a peripheral nerve should be suspected with the onset of unexplained neurological pain and sensory symptoms following surgery or injury. Peripheral nerve specialists working within a multi-professional team are well placed to undertake a thorough and systematic assessment, request appropriate investigations and formulate a bespoke management strategy encompassing non-surgical interventions. Non-responders or transient responders may require surgical intervention for the management of symptomatic neuromas and perioperative care should be with support from the multi-professional team.
Ethical approval
All images have been used with full publication consent from patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Authors contributions
TC and DMP were involved in paper design, TC, DMP and SB provided content and production of final draft. TC, SB, AN and DMP have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Ide C. Peripheral nerve regeneration. Neurosci Res 1996;25:101-21. [Google Scholar] |
| 2. | Bernstein DT, Hamilton KL, Foy C, Petersen NJ, Netscher DT. Comparison of magnification in primary digital nerve repair: Literature review, survey of practice trends, and assessment of 90 cadaveric repairs. J Hand Surg Am 2013;38:2144-50. [Google Scholar] |
| 3. | Jordaan P, Wang C, Ng C. Management of painful cutaneous neuromas around the wrist. Orthop Trauma 2017;31:290-5. [Google Scholar] |
| 4. | Mavrogenis AF, Pavlakis K, Stamatoukou A, Papagelopoulos PJ, Theoharis S, Zoubos AB, et al. Current treatment concepts for neuromas-in-continuity. Injury 2008;39 Suppl 3:S43-8. [Google Scholar] |
| 5. | Kretschmer T, Antoniadis G, Braun V, Rath SA, Richter HP. Evaluation of iatrogenic lesions in 722 surgically treated cases of peripheral nerve trauma. J Neurosurg 2001;94:905-12. [Google Scholar] |
| 6. | Terzis J, Faibisoff B, Williams B. The nerve gap: Suture under tension vs. Graft. Plast Reconstr Surg 1975;56:166-70. [Google Scholar] |
| 7. | Clark WL, Trumble TE, Swiontkowski MF, Tencer AF. Nerve tension and blood flow in a rat model of immediate and delayed repairs. J Hand Surg Am 1992;17:677-87. [Google Scholar] |
| 8. | Boyd BS, Puttlitz C, Gan J, Topp KS. Strain and excursion in the rat sciatic nerve during a modified straight leg raise are altered after traumatic nerve injury. J Orthop Res 2005;23:764-70. [Google Scholar] |
| 9. | Tay SC, Teoh LC, Yong FC, Tan SH. The prevention of neuroma formation by diathermy: An experimental study in the rat common peroneal nerve. Ann Acad Med Singapore 2005;34:362-8. [Google Scholar] |
| 10. | Li A, Meunier M, Rennekampff HO, Tenenhaus M. Surgical amputation of the digit: An investigation into the technical variations among hand surgeons. Eplasty 2013;13:e12. [Google Scholar] |
| 11. | Baranowski A. Classification of Chronic Pain. Revised, 2nd ed. Publication by International Society for the Study of Pain; 2011. [Google Scholar] |
| 12. | Strauch B, Lang A, Ferder M, Keyes-Ford M, Freeman K, Newstein D, et al. The ten test. Plast Reconstr Surg 1997;99:1074-8. [Google Scholar] |
| 13. | Torebjörk E. Human microneurography and intraneural microstimulation in the study of neuropathic pain. Muscle Nerve 1993;16:1063-5. [Google Scholar] |
| 14. | Patterson RW, Li Z, Smith BP, Smith TL, Koman LA. Complex regional pain syndrome of the upper extremity. J Hand Surg Am 2011;36:1553-62. [Google Scholar] |
| 15. | Scadding JW, Koltzenburg M, McMahon FB, Koltzenburg F, Tracey FM, Turk C. Painful peripheral neuropathies. In: Wall and Melzacks Textbook of Pain. 6th ed. Saunders; 2013. p. 926. [Google Scholar] |
| 16. | Heinen C, Dömer P, Schmidt T, Kewitz B, Janssen-Bienhold U, Kretschmer T, et al. Fascicular ratio pilot study: High-resolution neurosonography-A possible tool for quantitative assessment of traumatic peripheral nerve lesions before and after nerve surgery. Neurosurgery 2018. [Google Scholar] |
| 17. | Padua L, Liotta G, Di Pasquale A, Granata G, Pazzaglia C, Caliandro P, et al. Contribution of ultrasound in the assessment of nerve diseases. Eur J Neurol 2012;19:47-54. [Google Scholar] |
| 18. | Bergmeister KD, Schönle P, Böcker AH, Kronlage M, Godel T, Daeschler S, et al. Improved diagnostics and therapeutic decision making in traumatic peripheral nerve lesions using MR-neurography. Handchir Mikrochir Plast Chir 2018;50:232-40. [Google Scholar] |
| 19. | Kronlage M, Schwehr V, Schwarz D, Godel T, Uhlmann L, Heiland S, et al. Peripheral nerve diffusion tensor imaging (DTI): Normal values and demographic determinants in a cohort of 60 healthy individuals. Eur Radiol 2018;28:1801-8. [Google Scholar] |
| 20. | Lluch AL, Beasley RW. Treatment of dysesthesia of the sensory branch of the radial nerve by distal posterior interosseous neurectomy. J Hand Surg Am 1989;14:121-4. [Google Scholar] |
| 21. | Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol 2015;14:162-73. [Google Scholar] |
| 22. | Hansson PT, Attal N, Baron R, Cruccu G. Toward a definition of pharmacoresistant neuropathic pain. Eur J Pain 2009;13:439-40. [Google Scholar] |
| 23. | Sommer C, Cruccu G. Topical treatment of peripheral neuropathic pain: Applying the evidence. J Pain Symptom Manage 2017;53:614-29. [Google Scholar] |
| 24. | Derry S, Wiffen PJ, Moore RA, Quinlan J. Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst Rev 2014:CD010958. [Google Scholar] |
| 25. | Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M, et al. 5% lidocaine medicated plaster versus pregabalin in post-herpetic neuralgia and diabetic polyneuropathy: An open-label, non-inferiority two-stage RCT study. Curr Med Res Opin 2009;25:1663-76. [Google Scholar] |
| 26. | Mou J, Paillard F, Turnbull B, Trudeau J, Stoker M, Katz NP, et al. Efficacy of Qutenza® (capsaicin) 8% patch for neuropathic pain: A meta-analysis of the Qutenza clinical trials database. Pain 2013;154:1632-9. [Google Scholar] |
| 27. | Gálvez R, Navez ML, Moyle G, Maihöfner C, Stoker M, Ernault E, et al. Capsaicin 8% patch repeat treatment in nondiabetic peripheral neuropathic pain: A 52-week, open-label, single-arm, safety study. Clin J Pain 2017;33:921-31. [Google Scholar] |
| 28. | Göransson I, Cederlund R. A study of the effect of desensitization on hyperaesthesia in the hand and upper extremity after injury or surgery. Hand Ther 2010;16:12-8. [Google Scholar] |
| 29. | Rosén B, Balkeniu C, Lundborg G. Sensory re-education today and tomorrow: A review of evolving concepts. Hand Ther 2003;8:48-56. [Google Scholar] |
| 30. | Wynn Parry CB. Rehabilitation of the Hand. 2nd ed. London: Butterworths; 1966. [Google Scholar] |
| 31. | Rosén B, Vikström P, Turner S, McGrouther DA, Selles RW, Schreuders TA, et al. Enhanced early sensory outcome after nerve repair as a result of immediate post-operative re-learning: A randomized controlled trial. J Hand Surg Eur Vol 2015;40:598-606. [Google Scholar] |
| 32. | Rothgangel A, Braun S, de Witte L, Beurskens A, Smeets R. Development of a clinical framework for mirror therapy in patients with phantom limb pain: An evidence-based practice approach. Pain Pract 2016;16:422-34. [Google Scholar] |
| 33. | Rothgangel AS, Braun SM, Beurskens AJ, Seitz RJ, Wade DT. The clinical aspects of mirror therapy in rehabilitation: A systematic review of the literature. Int J Rehabil Res 2011;34:1-3. [Google Scholar] |
| 34. | Wittkopf PG, Johnson MI. Mirror therapy: A potential intervention for pain management. Rev Assoc Med Bras (1992) 2017;63:1000-5. [Google Scholar] |
| 35. | Laing T, Siddiqui A, Sood M. The management of neuropathic pain from neuromas in the upper limb: Surgical techniques and future directions. Plast Aesthetic Res 2015;2:165. [Google Scholar] |
| 36. | Melzack R, Wall PD. Pain mechanisms: A New Theory. Science. 1965;150(3699):971-9. [Google Scholar] |
Fulltext Views
4,296
PDF downloads
1,833





