Translate this page into:
The surgical management of traumatic neuromas
2 Consultant, Hand and Peripheral Nerve Surgeon, West Midlands Brachial Plexus and Peripheral Nerve Injury Service, Queen Elizabeth Hospital, Birmingham, UK
Corresponding Author:
Tom Challoner
Department of Plastic Surgery, Queen Elizabeth Hospital, Mindelsohn Way, Birmingham B15 2GW
UK
tom.challoner@gmail.com
| How to cite this article: Challoner T, Nijran A, Power DM. The surgical management of traumatic neuromas. J Musculoskelet Surg Res 2019;3:22-29 |
Abstract
Following injury to a peripheral nerve, a neuroma may form and cause severe debilitating neuropathic pain. End neuromas are the result of an amputation or are found at the proximal stump in a complete nerve transection or rupture. Neuromas-in-continuity follows traction or compression injury and may complicate repair of a nerve transection or rupture. Symptomatic neuromas should be assessed and treated by a peripheral nerve surgeon working within a multi-professional team. The objectives of the initial treatment should encompass pain management, psychological support and physical therapies with the aim of restoration of normal nerve response thresholds to afferent stimuli and optimisation of the perineuroma environment. Surgical intervention should be reserved for non-responders and in cases where reconstruction of nerve function is deemed essential for useful functional recovery and pain resolution.
Introduction
Neuromas may form at the site of a peripheral nerve injury. A disorganised mass of neural and connective tissue elements with lowered stimulation thresholds may predispose to mechanical irritation and spontaneous electrical discharge. The mechanical environment around a neuroma can cause tether resulting in movement-associated pain and in severe cases pseudoparalysis. Clinical assessment should aim to diagnose the injured nerve, the location of the neuroma and in cases of neuroma-in-continuity, the quality of any preserved function. Local anaesthetic nerve blocks are an essential step in the diagnostic pathway. Non-surgical interventions include pain management, physical therapies, neurorehabilitation, psychological support and neuromodulation.
Surgical intervention should be reserved for patients who fail to gain adequate symptom control with non-operative interventions or patients with a neuroma in continuity and no preserved distal function in a critical nerve that requires reconstruction.
Preparation to Surgery
Before considering surgery, a period of nonsurgical management is required. During this phase, the patient should be helped to understand their condition and treatment options, they should have their pain management optimised, physical and neurorehabilitation treatment modalities should be considered and the patient should be supported in developing robust coping mechanisms. The alternative treatment possibilities and attendant risks and potential benefits should be discussed in detail. A diagnostic nerve block can be used to identify the nerve of origin for the neuroma, assess peripheral pain responses and demonstrate the effects of a neurectomy should that technique be under consideration. The position of the neuroma should be marked on the skin before anaesthesia.
Surgical Techniques
Limb surgery is best accomplished with general anaesthesia or regional anaesthesia with a tourniquet to provide a bloodless surgical field. In a scarred bed, a nerve should be explored initially proximal and sometimes distal to the zone of injury to avoid further inadvertent damage. Where possible, dissection should proceed from proximal to distal along the course of a nerve to clearly define and avoid damage to side branches.
The technique employed in neuroma surgery is dependent on the type of neuroma, the position of the neuroma, the loss of function, soft tissue condition and the presence of a distal nerve stump in cases of nerve transection or rupture. End neuromas formed from transection or amputation can be treated with neurolysis, resection, proximal redirection, burying into deep tissues, capping, centrocentral coaptation with other nerves, targeted muscle reinnervation, fat grafting for scar management and soft tissue resurfacing or distal reconstruction with nerve grafts where a suitable distal stump remains. Neuroma-in-continuity results from traction injuries without rupture or following primary nerve repair or graft. In such cases, distal function should be critically assessed. Useful preserved function in a patient with neurostenalgia may be managed with neurolysis and possibly use of adjunctive nerve wrapping to prevent recurrent scar development. Absent distal function or minimal distal function can be managed with resection of the damaged nerve segment and reconstruction with nerve grafts or processed nerve allograft. Restoration of the nerve continuity is an important step in returning normal afferent sensory information to the sensory cortex and downregulating central sensitisation processes.
Neurolysis
Scar formation in the vicinity of an injured nerve may cause compression, ischaemia and impaired function. Adhesions from scar impair normal nerve gliding and create traction on the damaged area of nerve during normal physiological motion (neurostenalgia). Neurolysis should be performed with loupe magnification, no neuromuscular paralysis (for motor or mixed motor and sensory nerves) and using an intraoperative nerve stimulator to confirm stimulation thresholds before neurolysis and following neurolysis for motor or mixed nerves. The neurolysis is performed with sharp dissection with a scalpel blade along the junction between epineurium and scar. In cases where the epineurium is damaged and replaced by scar, a limited intraneural neurolysis is required and should be performed using microinstruments and an operating microscope. The nerve can be mobilised and then tagged with silicone elastic loops that can be used for gentle retraction without excessive handling of the nerve. Neurolysis is considered for painful nerves that have useful preserved function where excision and reconstruction of the damaged segment is contraindicated. However, the patient should be consented for excision and nerve grafting should the injury be severe, or in cases where there is damage to the nerve during neurolysis. A surgical neurolysis is a controlled injury to the nerve bed and will be followed by the formation of post-surgical scar. The optimum timing of the neurolysis is when the acute injury has settled and scar has commenced softening and remodelling. Careful haemostasis is helpful in avoiding further inflammation and scar after neurolysis. Tisseel™ (Baxter) tissue glue may be used to provide a barrier between the epineurium and the surgical bed. The fibrin clot is broken down and absorbed within a few weeks of surgery but may provide a temporary barrier to direct scar formation onto the nerve [Figure - 1].
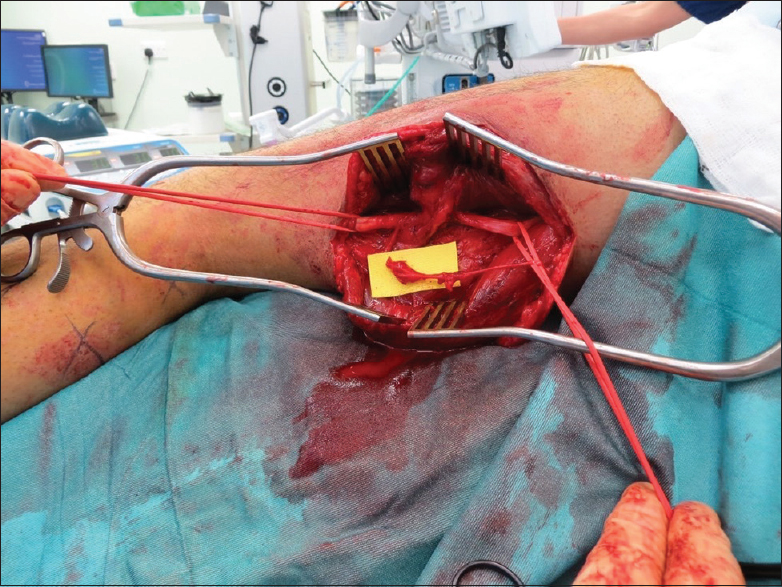 |
| Figure 1: Neuroma-in-continuity of the sciatic nerve with useful function after a stab in the thigh. Tether to biceps femoris treated with neurolysis |
Nerve wrapping
After neurolysis, if the bed is scarred, interposition of a barrier may help to prevent further direct scar adhesion to a nerve. There are a number of materials commercially available for this purpose. The Vivosorb®(Polyganics, Gronigen, Netherlands) is a synthetic polycaprolactone bioresorbable polymer sheet that can be loosely wrapped around and injured nerve and sutured in place acting as a mechanical barrier to scar formation. The polymer is converted to a hydrogel and undergoes resorption over 18 months.
The AxoGuard® nerve protector (AxoGen Inc., Alachua, Florida, USA) is a layered porcine small intestine submucosal collagen membrane that can be wrapped around injured nerves. The layer revascularises well and causes minimal soft tissue reaction. It is useful when there is epineural damage. Kokkalis et al.[1] demonstrated the material remodelled into a structure similar to epineurium and became revascularised, allowing nerve gliding, without electrophysical harm to the nerve [Figure - 2]a and [Figure - 2]b.
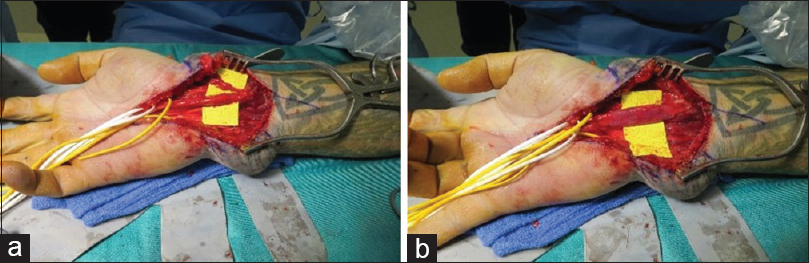 |
| Figure 2: (a and b) Neurolysis of the median and palmar branches of the median nerve after repair with AxoGuard®nerve protector wrap. (a) Repair of median and palmar branch of median nerve. (b) AxoGuard®wrap in situ |
Avive®(AxoGen Inc, Florida, USA) is a human umbilical cord amniotic membrane wrap that has been developed to resurface acutely injured nerves where there may be further reconstructive surgery planned. This is not indicated in the chronically injured nerve.
Biological tissue wraps may be locally harvested and rotated as vascularised adipose and fascial layers to wrap or resurface chronically scarred nerves. In the upper limb, the radial artery perforator flap can be elevated and rotated distally to cover the median nerve at the wrist of within the carpal tunnel. There is a risk of leaving the lateral cutaneous nerve of the forearm exposed or in scar and care should be exerted using this technique in patients with sensitisation. The ulnar artery perforator (Becker) flap can be used in a similar way.[2]
In situations where the skin is involved in the scar onto the nerve, consideration of resurfacing with an adipofascial cutaneous flap should be given. Alternative strategies for skin and soft tissue resurfacing include using tissue expansion or free tissue transfer.
One of the challenges with wrapping techniques is to avoid constriction or further tether points. Junctional scar formation at the point where a wrap meets normal tissues can lead to further tether points remote from the original neurostenalgia site.
Neuroma excision and reconstruction
In situations where there is a neuroma-in-continuity without useful distal function, also known as non-conducting neuroma, or an end neuroma from a nerve transection or rupture with a distal nerve stump available, debridement and nerve reconstruction is required. Intraoperative electrostimulation is useful in identifying non-conducting neuromas, which display a lack of electrical conductivity and poor end-motor responses. Once a diagnosis has been made, the non-conducting segment is excised and reconstructed as appropriate.
Simple excision of the neuroma is associated with high rates of recurrence and need for further surgical procedures.[3] Failure to restore the nerve continuity potentiates the central mechanism for nerve hypersensitivity. There will also be a functional deficit without reconstruction. Debridement of both the proximal and distal stumps should be undertaken using proper magnification and neurotomes until fascicle structure is identified with no significant interfascicular scar, revealing pouting of the proximal cut fascicles, and punctate bleeding [Figure - 3]a and [Figure - 3]b.
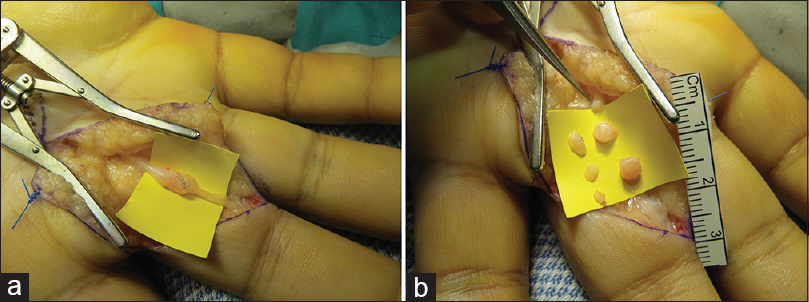 |
| Figure 3: (a and b) Serial debridement of a neuroma-in-continuity of the middle finger radial digital nerve following previous repair. (a) Neuroma demonstrated (b) multiple segments debrided to normal nerve |
Autograft remains the gold standard technique for restoring a nerve gap. Common autologous nerve graft donor nerves include the lateral and medial cutaneous nerves of the forearm in the upper limb and the sural nerve in the lower limb. Donor morbidity is predictable and the rate of symptomatic neuroma is estimated at between 3% and 5%. In large diameter nerves, several cables of donor autologous nerve are required to bridge the gap, and in such cases, the sural nerve is preferred due to the length available. The nerve is either sutured into position, held with tissue glue or a combination of both techniques. Wrapping the repairs may reduce the risk of neuroma-in-continuity at the coaptation sites at either end of the graft. The graft must be placed in loosely to prevent dehiscence and end neuroma formation. Autologous graft remains the gold standard for bridging gaps in critical sensory nerves and for mixed motor-sensory nerve reconstruction. In cases where there is an important motor function distal to the site of reconstruction, motor reinnervation using a distal nerve transfer may provide a more reliable method of reconstruction.
However, in individuals living with neuropathic pain, there is a risk of central sensitisation of the proximal donor nerve harvest site, with neuroma formation, tether in scar or new neuropathic pain. The risk must be balanced against the benefit of autologous nerve graft over other reconstructive options. In short gap (<12 mm) sensory reconstruction of a noncritical nerve, a conduit can be used to bridge the gap with reasonable functional results. Waitayawinyu et al.[4] tested both Type 1 collagen and polyglycolic acid conduits against autografts, finding comparable results with the collagen conduit and autograft for gaps up to 10 mm. Conduits, however, do not provide reliable nerve regeneration over long segments. Whitlock et al.[5] found segments over 12 mm were best reconstructed with isograft whereas for smaller segments under 12 mm, processed allograft was superior to conduit. Such circumstances are rare as there is typically a longer gap in all cases of delayed nerve reconstruction, whether end neuroma or neuroma-in-continuity.
AVANCE® processed nerve allograft (AxoGen Inc., Florida, USA) is a useful alternative to autologous nerve grafting in situ ations where the gap is in a non-critical nerve in a patient with sensitisation.
The processed nerve allograft is human nerve that is chemically decellularised and treated with enzymes to deplete neurotoxic proteoglycans, providing a scaffold for neural regeneration consisting of endoneurial tubes. No immunosuppression is required following implantation. The graft rapidly revascularises, and Schwann cells migrate from proximal and distal stumps to populate the graft and support neural regeneration. It is available in a range of diameters from 1–2 mm to 4–5 mm with variable lengths to a maximum of 70 mm. It can be used as a single graft or in a multiple cabled graft configuration for reconstruction of large diameter nerves [Figure - 4]a and [Figure - 4]b. The benefit of allograft is that the prerequisite size can be selected after adequate nerve debridement and there is no donor morbidity. The graft can be sutured in place, used with Tisseel™, or AxoGuard® collagen nerve connectors can be used to provide sutureless coaptation sites.
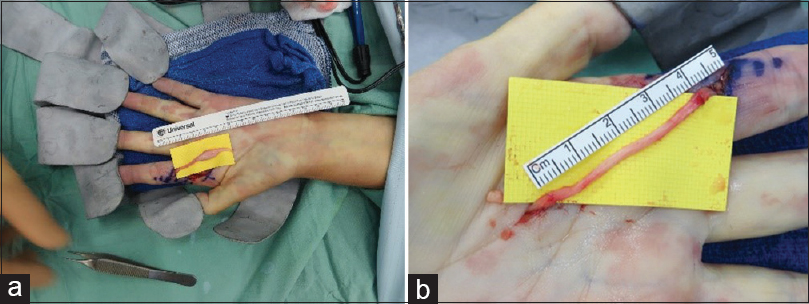 |
| Figure 4: (a and b) Reconstruction of a neuroma-in-continuity of the radial digital nerve of the index finger using AVANCE®processed nerve allograft. (a) Neuroma-in-continuity (b) allograft anastomosed in place |
The evidence to support the use of processed nerve allograft in nerve surgery is emerging. The argument for reconstruction of a painful neuroma in non-critical nerves with a distal stump available is a compelling one as there is no donor morbidity and reliable regeneration facilitating downregulation of central sensitisation mechanisms when peripheral regeneration is complete.
Moore et al. compared AVANCE® allografts to isografts (graft of tissue between two genetically identical individuals) and nerve conduits in a rat model,[6] demonstrating superiority over conduits, but supporting less neuronal regeneration than either isograft or acellular graft processed by an alternative cold-preservative technique. Whitlock et al.[5] also compared isografts with AVANCE®, in a rat model, suggesting that for short gaps up to 14 mm, the allograft was comparable to isograft after 12 weeks; however, in longer gaps of 28 mm, isograft was superior.
Brooks et al.[7] analysed the outcomes from a multicentre study of AVANCE®, finding 87% of patients showed meaningful recovery in gaps of between 5 mm and 50 mm. Cho et al.[8] in a retrospective analysis also reported superiority to conduits, no side effects, meaningful levels of recovery in 89% of digital nerve repairs and 75% of median nerve repairs, similar to those reported with autograft. They also suggested allografts provided recovery in nerve gaps of between 5 mm and 50 mm.
While these studies report on functional outcomes in the reconstruction of functional nerves, which is applicable to both end neuromas with a distal stump present and neuromas-in-continuity, they also demonstrate the growth of nerve endings into the allograft, which could be used to direct axonal growth away from damaged skin in amputation stumps and into muscle if further length is required to achieve this.
Nerve relocation
Following excision of an end neuroma if there is no distal stump available, the proximal nerve stump can be mobilised and relocated proximally into deeper tissues, typically muscle or bone. The aim of this technique is to prevent mechanical irritation of the nerve by recurrent tether in scar and to protect it from direct trauma; this should be outside the zone of injury to prevent further scarring.
Several techniques have been described for burying the proximal nerve stump. Moszkowicz first reported success with intramuscular burying in 1918.[9] Dellon and MacKinnon[10] demonstrated a significant reduction in nerve regeneration when buried in muscle in a primate model compared to control, possibly reducing excitability of the nerve end, with subsequent subjective pain relief in 81%. This technique is performed by securing the proximal nerve stump deep within a local muscle with adequate bulk.[11] The new neuromuscular interface is poorly understood. New neuromuscular stimulation may be possible though, either motor axons from a mixed nerve neuroma or from autonomic cholinergic fibres in sensory nerves, causing spontaneous depolarisation and muscle contraction, which may be painful if there is nerve tether [Figure - 5].
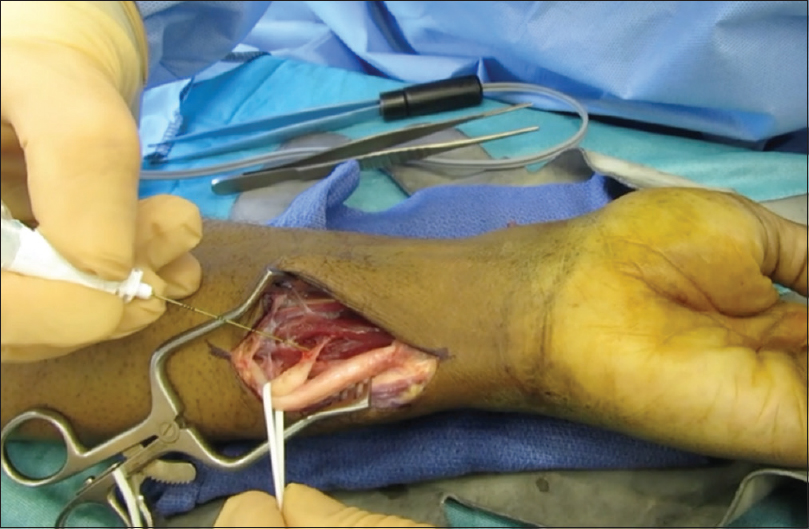 |
| Figure 5: A neuroma of the dorsal branch of the ulnar nerve relocated to the flexor digitorum superficialis |
As the nerve must be brought proximally, this can lead to sacrifice of functionally normal branches to accommodate reaching the desired muscle, leading to further motor or sensory defects. This would provide an unacceptable compromise; therefore, the end neuroma stump can be lengthened with allograft to provide sufficient length for relocation. The allograft provides a scaffold for supported neural regeneration; however, without maintained neurotrophic stimulation, the axonal regeneration will ultimately fail. The concept is described as a 'graft to nowhere' [Figure - 6].
 |
| Figure 6: (a and b) Allograft proximal relocation of a neuroma in the palm to pronator quadratus after resection of a hypoplastic thumb and index pollicisation |
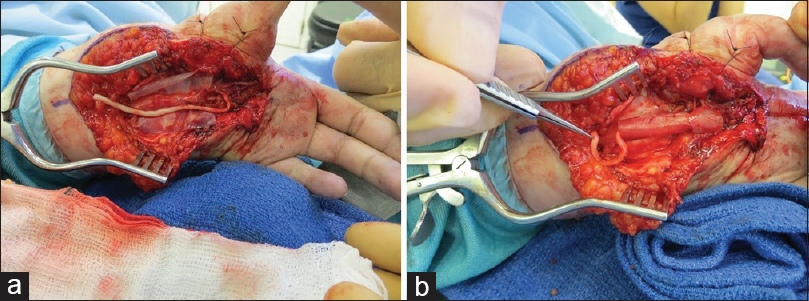
The allograft can alternatively be secured in denervated muscle or if the neuroma is in an amputated limb, it can be sutured to a sectioned motor branch from a nearby nerve creating an opportunity for reinnervation from the injured nerve. This technique is known as targeted muscle reinnervation (TMR) and is useful in cases of amputation with phantom limb pain [Figure - 7].
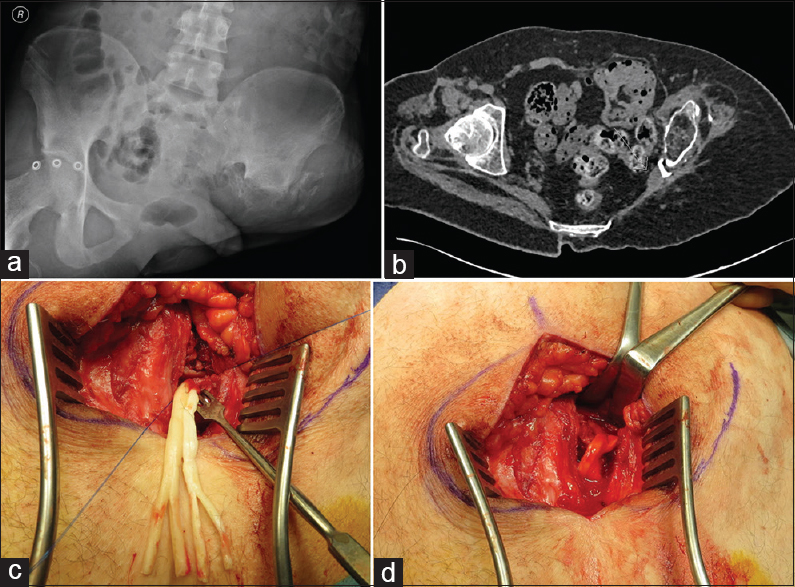 |
| Figure 7: (a-d) Nerve allograft redirection of a sciatic nerve neuroma from the greater sciatic notch to the gluteus medius muscle in a high transpelvic amputation following blast injury from an improvised explosive device. (a) Pelvic radiograph with heterotopic bone at the site of left pelvis resection. (b) Axial computed tomography scan with sciatic nerve stump adjacent to heterotopic bone. (c) AVANCE®processed nerve allograft lengthening of sciatic nerve after neuroma excision. (d) Allograft implantation to the gluteus medius muscle at multiple sites |
Intraosseous burying of nerve endings was first described by Boldrey in 1943. It is more technically challenging and requires a large dissection, potentially creating more scar tissue. A hole is drilled into the cortex of the desired bone at a site where there is limited mobility. A suture is then passed through the nerve end and through the bone, pulling the nerve into the medulla.[12] The suture is tied in place to a bolster on the contralateral cortex. An alternative is direct placement into a cortical drill hole and judicious suture placement to the periosteum or Tisseel™ application to prevent secondary displacement. These techniques can lead to further scar tissue formation and subsequent tether points.
An animal model burying femoral nerve neuromas into the tunica adventitia of the femoral vein demonstrated a statistically significant reduction in neuroma size;[13] however, this is more technically challenging and is not superior to the above-mentioned techniques.
Neurectomy and chemical axonotmesis
Simple neuroma excision without neuroma relocation poses a risk of recurrent neuroma formation in the original scarred bed with subsequent tether and recurrence of neuropathic pain. Following injury to the superficial radial nerve, dysaesthesia in the radial nerve territory may persist despite a proximal sectioning of the superficial radial nerve. While it is possible that the perception of unpleasant sensation is through cutaneous overlap with hypersensitivity expressed in the lateral cutaneous nerve of the forearm, Lluch and Beasley[14] postulated that because a high radial nerve injury has no such dysaesthesia; it is possible that the aberrant sensory pathway is perceived through the posterior interosseous nerve (PIN) and successful management of the dysaesthesia was achieved through distal PIN sectioning above the wrist.
The use of nerve blocks preoperatively in a staged fashion can identify unusual aberrant pathways and provide targets for surgical resection.
Gruber et al.[15] described a small series of ultrasound-guided phenol injections for painful neuromas. Phenol, glycerol and steroid have all been described with the aim of axonal disruption. Phenol and glycerol cause demyelination, axonal degeneration and subsequently, disruption of axonal architecture; however, the high viscosity of glycerol makes it less effective as injecting is more difficult. Steroid injection has been tried to reduce inflammation and scarring surrounding the nerve, potentially a contributing factor in pain. Alcohol although effective in nerve disruption is toxic to surrounding tissues and can lead to painful neuritis. Ultrasonography can be used to guide the intraneural injection and prevent adjacent damage.
Nerve capping
Covering the cut surface of a proximal nerve stump after neuroma resection may prevent tether of the regenerating axons in the scar. This technique can be used in combination with proximal relocation or in isolation, where relocation may result in damage to side branches so that nerve end is capped in situ [Figure - 8]. The soft tissue envelope must be adequate at the site of capping.
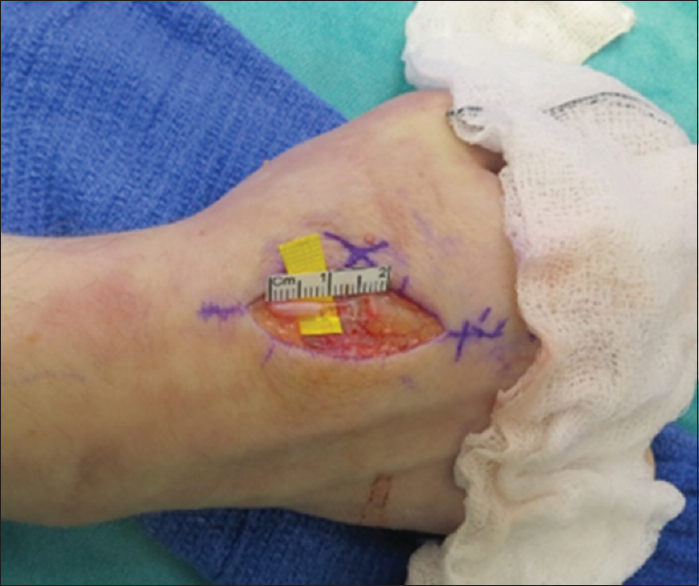 |
| Figure 8: Neurocap® application to a superficial radial neuroma |
Capping of a nerve can be performed using either biological tissues or synthetic materials. Silicone caps were first described in 1977 by Swanson et al.[16] and were employed when the distal nerve stump was not available for neurorrhaphy or in a situation where the lost distal function was not deemed critical. They reported 15 of 18 patients were relieved of their symptoms as a result. The cap may cause mechanical irritation in the long term.
Polyganics (Groningen, Netherlands) have developed a bioresorbable polycaprolactone Neurocap® to suture over the proximal nerve stump after neuroma resection, preventing the need for burying in proximal tissues. The encasement of the nerve ending prevents axon regeneration into scar. There is a small chamber to allow unsupported neural regeneration. Blocking the nerve from the bed prevents the mechanical irritation that renders a neuroma symptomatic. The cap undergoes hydrolysis and forms a gel around the nerve after 12 weeks; then, full resorption is achieved by 18 months. The resorption reduces the risk of long-term mechanical irritation [Figure - 9].
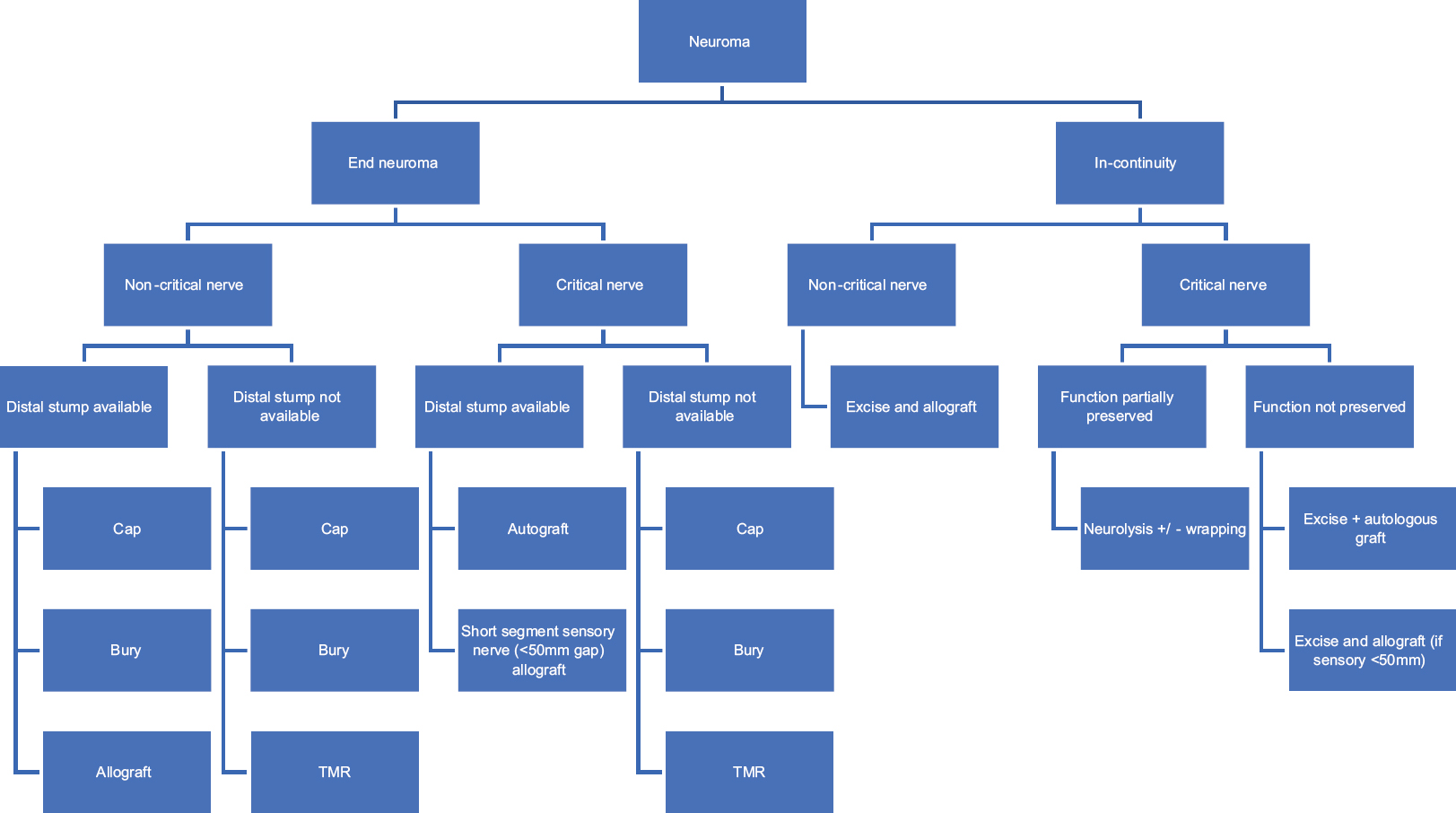 |
| Figure 9: A suggested algorithm for decision-making in neuroma management |
Fat grafting
Vaienti et al.[17] described using fat harvested using the Coleman technique to inject into the affected area following excision of a neuroma. The fat is postulated to improve gliding, protect the nerve stump from mechanical trauma and potentially provide adipose-derived stem cells to the scar milieu. This technique negates the potential donor site morbidity of autologous grafting. Mean pain scores were reduced by 23% in 7 out of 7 patients managed by this technique; however, in the absence of controls, it is not known what role the fat graft plays versus resection of neuroma alone.
Targeted muscle reinnervation
Symptomatic neuromas occur following lower limb amputation in-between 13% and 32% of cases.[18] Kuiken et al.[19] described a technique for joining the affected mixed motor and sensory nerve to a healthy motor nerve supplying a portion of functionally expendable muscle in the vicinity of the amputation stump. The theory is that the divided axons regenerate along the motor nerve, providing a more normal physiological afferent stimulus, which causes central downregulation of the hypersensitivity and diminution of the neuropathic pain, rather than forming another symptomatic neuroma. Pet et al.[18] published retrospective data on primary TMR to prevent neuroma formation at amputation versus secondary TMR as a treatment for painful neuromas following amputation. They reported 92% of primary TMR and 87% of secondary TMR were free neuroma pain following the intervention. When used for proximal muscle reinnervation for prosthetic control, direct muscle implantation of split proximal nerve stumps shows promise in the reduction of phantom and neuroma pain.
Neuroma Surgery Decision-Making
A 2018 meta-analysis of the surgical treatment of neuroma pain was performed by Poppler et al.[20] Surgical treatment, including excision, excision and proximal relocation, excision and cap, excision and repair or neurolysis and coverage, was effective in 77% of patients. No significant difference was found between techniques. In patients with pain for over 24 months, and >2 previous operations, excision and relocation or neurolysis were significantly more likely to improve symptoms than other surgical techniques. A diagnosis of complex regional pain syndrome associated with a nerve injury (Type 2) is not an absolute contraindication to neuroma surgery. Careful evaluation and optimisation of pain with the involvement of the multi-professional team are required if surgery is going to achieve a successful outcome in such cases.
Post-Operative Pain Management
Optimisation of pain management in the perioperative period is essential to achieving a successful outcome following neuroma surgery. Local anaesthetic field blocks or regional anaesthetic blocks are excellent for early pain management; but, to provide extended relief, an indwelling nerve catheter positioned at surgery adjacent to the operated nerve, proximal to the neuroma resection can maintain relief with a background infusion and intermittent bolus administration. Patients should continue neuromodulatory agents and normal analgesia during the first few weeks after surgery and wean when the inflammation at the operative site has settled.
The Future of Neuroma Prevention and Treatment
Extensive research has been performed to ascertain the ideal method in which to repair a nerve, prevent neuroma and manage painful neuromata. Barton et al. described suture-less methods of nerve repair, eliminating foreign body reaction, reducing further trauma to the nerve ending and avoiding nerve end distortion.[21] Fibrin glues may be used as adjuncts to primary nerve repair, reducing suture utilisation but not having sufficient tensile strength to overcome the strain inherent at a nerve repair site due to tensegrity. When using an interposition graft, the strain can be mitigated with a longer graft, and in such a situation, fibrin glue may be adequate in preventing repair site dehiscence. Tisseel™ (Baxter Healthcare, Illinois, USA), physiologically resembles a blood clot, which encases the nerve and holds the two endings together. Sameem et al.[22] reviewed animal studies finding Tisseel™ easier, and quicker to apply, with equal or superior results compared to microsurgical repair.
Bridging damaged nerves with conduits shows promise, and using a conduit to allow a detensioned gap in a primary nerve repair may prevent the complications of sutures without significantly reducing the quality or axon regeneration. The future of nerve surgery is likely to involve suture-less repairs and laser tissue welding techniques,[21] polymer glues and nerve connectors with local drug delivery to modulate the repair environment. Connector-assisted repairs and adhesive repairs are simpler to master than microsurgical suture repair, producing consistently high-quality repairs without creating secondary damage to the nerve ends.
Reducing repair site scar and improving alignment will be critical to the success of axon fusion nerve repair using hydrogels[23] that will allow axons to repair before the onset of Wallerian degeneration and thereby reducing the disorganised neural regeneration in the scar that signifies a repair site neuroma.
Molecular neurosurgery refers to the use of neurotoxic substances to destroy specific types of neurons. Suicide transport is the uptake an axonal transport that produces a neural lesion afferent to the site of toxin injection.[24] Various substances have been trialled to induce neuronal damage including doxorubicin and ricin, which are relatively non-specific. Therefore, an anti-neuronal immunotoxin conjugate was developed combining an anti-Thy-1 monoclonal antibody, OX7 and saporin, a ribosome-inactivating protein similar to ricin. Thy-1 is expressed on all adult neurons, and as the OX7-saporin molecule produces little systemic toxicity, it is an excellent choice for suicide transport. Mavrogenis et al. demonstrated inhibition of neuroma-in-continuity formation as a result of OX7-saporin injection compared to control in rats, however, also damaging healthy neurons. Further research is required to improve specificity of the toxin delivery and neuroma targeting.[25]
Conclusion
Following injury to a peripheral nerve, symptomatic neuromas may form at the site of nerve injury or following repair. A systematic approach to diagnosis with the involvement of a multi-professional team is the key to achieving a successful resolution. Non-operative strategies should be tried before considering any surgical intervention. The procedure offered should consider the extent of injury, retained function of the affected nerve, the importance of the function in the affected nerve, the degree of sensitisation, the possibility of distal reconstruction and the patient's expectations and resilience to cope with the post-operative management pathway. Patients must be involved in the decision-making process and be made aware of the uncertainty regarding the surgical findings and therefore the different reconstruction possibilities and expected outcomes. The surgical management of neuromas is challenging and yet can be one of the most rewarding fields in peripheral nerve surgery, eliminating pain and restoring function to troubled patients living with chronic neuropathic pain.
Ethical approval
All images used with publication level consent from patients (or words to equivalent).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Author's contributions
TC performed the literature search, wrote the initial and final draft with editing from DMP and AN. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Kokkalis ZT, Pu C, Small GA, Weiser RW, Venouziou AI, Sotereanos DG, et al. Assessment of processed porcine extracellular matrix as a protective barrier in a rabbit nerve wrap model. J Reconstr Microsurg 2011;27:19-28. [Google Scholar] |
| 2. | Samson D, Power DM. The adipofascial radial artery perforator flap: A versatile reconstructive option in upper limb surgery. Hand Surg 2015;20:266-72. [Google Scholar] |
| 3. | Guse DM, Moran SL. Outcomes of the surgical treatment of peripheral neuromas of the hand and forearm: A 25-year comparative outcome study. Ann Plast Surg 2013;71:654-8. [Google Scholar] |
| 4. | Waitayawinyu T, Parisi DM, Miller B, Luria S, Morton HJ, Chin SH, et al. Acomparison of polyglycolic acid versus type 1 collagen bioabsorbable nerve conduits in a rat model: An alternative to autografting. J Hand Surg Am 2007;32:1521-9. [Google Scholar] |
| 5. | Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DA, Magill CK, et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve 2009;39:787-99. [Google Scholar] |
| 6. | Moore AM, MacEwan M, Santosa KB, Chenard KE, Ray WZ, Hunter DA, et al. Acellular nerve allografts in peripheral nerve regeneration: A comparative study. Muscle Nerve 2011;44:221-34. [Google Scholar] |
| 7. | Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, et al. Processed nerve allografts for peripheral nerve reconstruction: A multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery 2012;32:1-14. [Google Scholar] |
| 8. | Cho MS, Rinker BD, Weber RV, Chao JD, Ingari JV, Brooks D, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am 2012;37:2340-9. [Google Scholar] |
| 9. | Wu J, Chiu DT. Painful neuromas: A review of treatment modalities. Ann Plast Surg 1999;43:661-7. [Google Scholar] |
| 10. | Dellon AL, Mackinnon SE. Treatment of the painful neuroma by neuroma resection and muscle implantation. Plast Reconstr Surg 1986;77:427-38. [Google Scholar] |
| 11. | Jordaan P, Wang C, Ng C. Management of painful cutaneous neuromas around the wrist. Orthop Trauma 2017;31:290-5. [Google Scholar] |
| 12. | Goldstein SA, Sturim HS. Intraosseous nerve transposition for treatment of painful neuromas. J Hand Surg Am 1985;10:270-4. [Google Scholar] |
| 13. | Low CK, Chew SH, Song IC, Ng TH. Implantation of a nerve ending into a vein. Clin Orthop Relat Res 2000;(379):242-6. [Google Scholar] |
| 14. | Lluch AL, Beasley RW. Treatment of dysesthesia of the sensory branch of the radial nerve by distal posterior interosseous neurectomy. J Hand Surg Am 1989;14:121-4. [Google Scholar] |
| 15. | Gruber H, Kovacs P, Peer S, Frischhut B, Bodner G. Sonographically guided phenol injection in painful stump neuroma. AJR Am J Roentgenol 2004;182:952-4. [Google Scholar] |
| 16. | Swanson AB, Boeve NR, Lumsden RM. The prevention and treatment of amputation neuromata by silicone capping. J Hand Surg Am 1977;2:70-8. [Google Scholar] |
| 17. | Vaienti L, Gazzola R, Villani F, Parodi PC. Perineural fat grafting in the treatment of painful neuromas. Tech Hand Up Extrem Surg 2012;16:52-5. [Google Scholar] |
| 18. | Pet MA, Ko JH, Friedly JL, Mourad PD, Smith DG. Does targeted nerve implantation reduce neuroma pain in amputees? Clin Orthop Relat Res 2014;472:2991-3001. [Google Scholar] |
| 19. | Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int 2004;28:245-53. [Google Scholar] |
| 20. | Poppler LH, Parikh RP, Bichanich MJ, Rebehn K, Bettlach CR, Mackinnon SE, et al. Surgical interventions for the treatment of painful neuroma: A comparative meta-analysis. Pain 2018;159:214-23. [Google Scholar] |
| 21. | Barton MJ, Morley JW, Stoodley MA, Lauto A, Mahns DA. Nerve repair: Toward a sutureless approach. Neurosurg Rev 2014;37:585-95. [Google Scholar] |
| 22. | Sameem M, Wood TJ, Bain JR. A systematic review on the use of fibrin glue for peripheral nerve repair. Plast Reconstr Surg 2011;127:2381-90. [Google Scholar] |
| 23. | Bittner GD, Sengelaub DR, Trevino RC, Peduzzi JD, Mikesh M, Ghergherehchi CL, et al. The curious ability of polyethylene glycol fusion technologies to restore lost behaviors after nerve severance. J Neurosci Res 2016;94:207-30. [Google Scholar] |
| 24. | Mavrogenis AF, Pavlakis K, Stamatoukou A, Papagelopoulos PJ, Theoharis S, Zoubos AB, et al. Current treatment concepts for neuromas-in-continuity. Injury 2008;39 Suppl 3:S43-8. [Google Scholar] |
| 25. | Mavrogenis AF, Pavlakis K, Stamatoukou A, Papagelopoulos PJ, Theoharis S, Zetahang Z, et al. Intraneural OX7-saporin for neuroma-in-continuity in a rat model. Eur J Orthop Surg Traumatol 2013;23:263-72. [Google Scholar] |
Fulltext Views
6,378
PDF downloads
743





